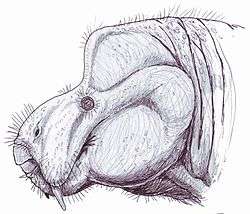
Aulacephalodon
Aulacephalodon is an extinct genus of medium-sized dicynodonts, or non-mammalian synapsids, that lived during Permian period, about 299-252 million years ago. Individuals of Aulacephalodon are commonly found in the Lower Beaufort Group of the Karoo Supergroup of South Africa and Zambia. Rising to dominance during the Late Permian, Aulacephalodon were the dominant terrestrial vertebrate herbivores until they became extinct during the Triassic.[1] No living relatives of Aulacephalodon exist today. Two species have been named, the type species, A. bainii, and a second species, A. peavoti. However, debate exists among paleontologists if A. peavoti is a true member of the genus Aulacephalodon.[1] Therefore, a majority of the information known about Aulacephalodon is in reference to discoveries about A. bainii.
| Aulacephalodon | |
|---|---|
 | |
| Skeleton of A. peavoti in the Field Museum of Natural History | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Therapsida |
| Clade: | †Dicynodontia |
| Family: | †Geikiidae |
| Genus: | †Aulacephalodon Seeley, 1898 |
| Type species | |
| Aulacephalodon bainii Seeley, 1898 (type) | |
| Species | |
| Synonyms | |
| |
The name Aulacephalodon combines the Greek words aulak- (aulax), meaning "a furrow", kephale, meaning "head," and odon, meaning tooth. Together, Aulacephalodon means "furrow-head tooth." The species Aulacephalodon bainii was named in honor of Andrew Geddes Bain (1797-1864), a Scottish geologist and road engineer who is credited with discovering the first dicynodont skull in South Africa.
Aulacephalodon belong to the family Geikiidae, a family of dicynodonts generally characterized by their short, broad skulls and large nasal bosses.[2] Two autamorphies used to define Geikiidae include (1) the posterior portion of the zygomatic arch thickened and/or downturned; (2) the labial fossa present.[2] Both features are found in Aulacephalodon and allow for suggestions to be made about possible sexual dimorphism evident in A. bainii.
Among certain anomodont therapsids, Aulacephalodon and Pelanomodon are distinguished from their related genera, Oudenodon and Rhachiocephalus by the specialization of their biting mechanism and the relative size of their nasal and prefrontal bosses.[3] Aulacephalodon and Pelanomodon bit using the transverse anterior tips of the jaws, while Oudenodon and Rhachiocephalus bit using the sides of their horn covered jaws.[3] The difference in jaw morphology between the two groups of genera is a notable dichotomy among these related therapsids. Aulacephalodon was unique among dicynodonts and its related therapsids because members of this genus were one of the only dicynodonts to possess canine tusks.[3]
Description
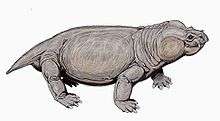
Aulacephalodon is considered to be medium-sized relative to other dicynodont species, unique to other dicynodont species due to the canine tusks they possessed.[1] Fossilization tends to have preserved only skulls and complete or fragmented bones of Aulacephalodon bainii, requiring paleontologists to use the unique features of the cranium when identifying specimens believed to belong to the genus. Aulacephalodon had short, broad skulls with a recorded range of 135 mm to 410 mm.[4] Comparison of juvenile and mature individuals suggest that Aulacephalodon demonstrated a positive allometric growth pattern for their cranial features and a negative allometric growth pattern for their tusk and orbit size as ontogenetic age increases.[4] Disproportionately large eyes in juveniles of a species is considered a characteristic of higher vertebrates.[4] Diagnostic features of Aulacephalodon include (1) the size of their nasal bosses, (2) the shape and articulation of cranial bones, and (3) the length-breadth ration of the skull.[4] The most complete restoration of Aulacephalodon is a skeleton of A. peavoti from the Field Museum of Natural History in Chicago.
| Wikimedia Commons has media related to Aulacephalodon. |
A set of footprints was found in the Teekloof Formation of the Beaufort Group by C.S. MacRae (1990), believing to have been made by either Aulacephalodon or the related Rhachiocephalus.[5] Biostratigraphy placed the formation that contained the tracks within the Cistecephalus Assemblage Zone.[5] Two sets of tracks were found, most likely belonging to two different individuals, with a pace approximately 600–650 mm in length.[5] The organism that made the tracks was a large, and heavily padded, homopodus quadruped with a short tail.[5] The tracks had a width of 800 mm, which when combined with the short stride length suggests Aulacephalodon, or Rhaciocephalus, was an inefficient walker.[5]
Discovery and naming
Origins of Aulacephalodon
Controversy over the number of species belonging to the genus Aulacephalodon has existed since the first specimens were discovered. The majority of specimens found were fragments, making proper diagnosis difficult for many of the specimens. Owen (1844) first described members of this genus as Dicynodon bainii, with five more species of dicynodont being discovered in subsequent years. Seeley (1898) divided Dicynodon into two subgenera and created the subgenus Aulacephalodon, suggesting all dicynodonts with a short snout and wide skull should be included in the subgenus. Two additional dicynodont species were discovered by Broom in 1912 and 1913. Broom (1921) also proposed a new subgenus of Dicynodon, Bainia, to describe tusked members of Dicynodon. When Broom finally recognized Seeley's subgenus, Aulacephalodon, as a valid genus in 1932. However, the spelling was altered to Aulacocephalodon. The incorrect genus Aulacocephalodon was used for many years until the correct spelling was pointed out by Keyser (1969). At least 17 species have been described as members of Aulacephalodon, however it is noted many of the features used to distinguish between the different species are size-dependent and highly susceptible to distortion. This has resulted in Aulacephalodon bainii being recognized as the type species of the genus, with the previous 17 species described as synonymous members of the species at various stages of growth.[4]
Aulacephalodon bainii and Aulacephalodon peavoti
When Aulacephalodon peavoti was first described, the cranium of one specimen was compared to Aulacephalodon bainii to determine if A. peavoti can accurately be described as Aulacephalodon. The most distinct difference between A. bainii and A. peavoti is that specimens of A. peavoti are not found to possess tusks, which is a notable feature of A. bainii and Aulacephalodon. A. peavoti is also found to have a wider and more upright scapula blade compared to A. bainii, with deeper fossa on the proximal end of the scapula. While both species share some similar post-cranial features, there are numerous differences in the shapes of various girdle and forelimb elements prevents paleontologists from definitively recognizing A. peavoti as a member of Aulacephalodon.[1]
Paleobiology
Diet
Aulacephalodon skulls show the transverse anterior tip of their short snout is reinforced by the palatal ridges and ridges on the snout, resulting in biting action restricted to the tip of their jaws.[4] The restricted jaw motion and presence of a horny beak structure suggests that Aulacephalodon were herbivores.[4] Based on the arid climate of the Cistecephalus Zone, Aulacephalodon is thought to have fed on the stems of woody plants present in the region.[6] The most commonly occurring plants in the region, Schizoneura and Phyllotheca, would have been available to Aulacephalodon to feed on.[4] Tollman et al. (1980) suggests that as a function of their ontogenetically increasing size, the breadth of their occupied niche increased throughout their life that resulted in changes to the size and type of food Aulacephalodon obtained.[4] A similar phenomenon is observed in modern genera Alligator and Crocodylus.[4]
Sexual dimorphism

Tollman et al. (1980) suggests that the nasal bosses in the skulls of collected Aulacephalodon specimens provide the most compelling evidence of sexual dimorphism in Aulacephalodon fossils.[4] Morphological studies found that the sizes of the nasal bosses of collected specimens can be categorized into three different size ranges, with specimens possessing the largest sized nasal bosses assumed to be mature males.[4] Female Aulacephalodon were found to be specimens with weakly developed nasal bosses.[4] Specimens that were thought to represent mature males also had larger craniums with a thicker squamosal at the border of the zygomatic arch.[4] Female specimens showed no thickening in the zygomatic arch.[4] The canine tusks also appear to be longer and further apart in male specimens.[4] Tollman et. all suggests sexual dimorphism existed in Aulacephalodon as a means of sexual selection and as a display mechanism.[4] Important limitations to this analysis are that no specimen showed entirely male or female characters and many of the specimens used were characterized as immature juveniles.[4] Possible explanations for this observation are that non-dimorphic characters present in the crania effectively mask the sexual dimorphism characters, or sexual dimorphic characters were not present in the fossils due to a lack of measurable characters.[4]
Paleoecology
Aulacephalodon specimens have been found in both the Madumabisa Mudstone "Formation" of Zambia and the Cistecephalus zone sediments of South Africa. Though geographically different localities, the specimens found at both locations are not found to be morphologically distinct from each other.[4] The terrain of the Cistecephalus zone is thought to be a lowland composed of vast floodplains, with many traversing streams that crossed the landscape.[7] The climate of the Beaufort Group during the Late Permian was semi-arid, with seasonal rainfall.[8] While the overall climate of the Cistecephalus zone was not suitable for diverse environments, this zone was the height of genetic diversity of dicynodonts.[6] Aulacephalodon occupied over 86% of the rich localities of the Cistecephalus zone, suggesting the habitat during this time was extremely suitable for Aulacephalodon.[6] Recent biostratigraphy of the Beaufort Group has marked the lower boundary of the Cistecephalus assemblage zone by the first appearance of Aulacephalodon and the first appearance of Cistecephalus.[9] This more specific boundary is the result of a refined biozonation map and new technology that allows for more accurate geographic information data (GIS).[9]
Possible explanation for extinction
Some paleontologists suggest that the end-Permian tetrapod extinctions, including Aulacephalodon, may have been caused by lowered atmospheric oxygen concentrations, resulting in both environmental and organismal hypoxia. The increase in size of the internal nares and secondary palate would decrease the respiratory efficiency of large anomodont therapsids, such as Aulacephalodon. Comparison of Triassic and late-Permian therapsids shows an increase in separation between the buccal and nasal cavities and larger internal nares in Triassic therapsids, demonstrating an increased efficiency of oxygen transport in a lower-oxygen environment. An important caveat to this explanation for extinction is that hypoxia is most likely one of many compounding environmental factors that led to the end-Permian tetrapod extinction.[10]
References
- Govender, R. (2008). "Description of the postcranial anatomy of Aulacephalodon baini and its possible relationship with "Aulacephalodon peavoti"" (PDF). South African Journal of Science. 104 (11–12): 479–486. doi:10.1590/s0038-23532008000600023. ISSN 0038-2353.
- Kammerer, Christian F., and Kenneth D. Angielczyk. "A proposed higher taxonomy of anomodont therapsids." Zootaxa 2018 (2009): 1-24.
- Keyser, A. W. "A re-evaluation of the systematics and morphology of certain anomodont Therapsida." (1972).
- Tollman, S. M., F. E. Grine, and B. D. Hahn. Ontogeny and sexual dimorphism in Aulacephalodon (Reptilia, Anomodontia). South African Museum, 1980.
- MacRae, C. S. "Fossil vertebrate tracks near Murraysburg." Cape Province: Palaeontologia africana 27 (1990): 83-88.
- King, G.M. (1986). "Distribution and Diversity of Dicynodonts: An Introductory Approach". Modern Geology. 10: 109–120.
- Yemane, K., and K. Kelts. "A short review of paleoenvironments for Lower Beaufort (Upper Permian) Karoo sequences from southern to central Africa: A major Gondwana Lacustrine episode." Journal of African Earth Sciences (and the Middle East) 10.1-2 (1990): 169-185.
- Smith, R. M. H. "A review of stratigraphy and sedimentary environments of the Karoo Basin of South Africa." Journal of African Earth Sciences (and the Middle East) 10.1-2 (1990): 117-137.
- van der Walt, Merrill, et al. "A new GIS-based biozone map of the Beaufort Group (Karoo Supergroup), South Africa." (2010).
- Angielczyk, Kenneth D., and Melony L. Walsh. "Patterns in the evolution of nares size and secondary palate length in anomodont therapsids (Synapsida): implications for hypoxia as a cause of end-Permian tetrapod extinctions." Journal of Paleontology 82.3 (2008): 528-542.