Amavadin
Amavadin is a vanadium-containing anion found in three species of poisonous Amanita mushrooms: A. muscaria, A. regalis, and A. velatipes.[1] Amavadin was first isolated and identified in 1972 by Kneifel and Bayer.[2] This anion, which appears as a blue solution, is an eight-coordinate vanadium complex.[1] A Ca2+ cation is often used to crystallize amavadin to obtain a good quality X-ray diffraction.[1] Oxidized amavadin can be isolated as its PPh4+ salt. The oxidized form contains vanadium(V), which can be used to obtain an NMR spectrum.[3]

 | |
 | |
| Names | |
|---|---|
| IUPAC name
bis[N-[(1S)-1-(carboxy-κO)ethyl]-N-(hydroxy-κO)-L-alaninato(2-)-.κN,κO]-vanadium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| [V{NO[CH(CH3)CO2]2}2]2− | |
| Molar mass | 398.94 g/mol |
| Appearance | Light blue in solution |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
The formation of amavadin begins with the formation of two tetradentate ligands.[3]
- 2 HON(CH(CH3)CO2H)2 + VO2+ → [V{NO[CH(CH3)CO2]2}2]2− + H2O + 4 H+
Structure and properties
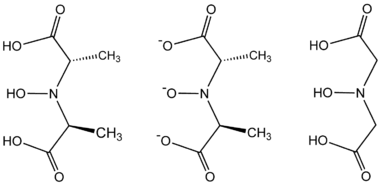
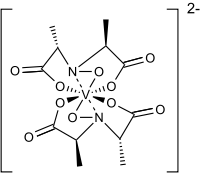
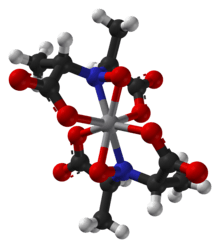
The ligand found in amavadin was first synthesized in 1954.[4] Amavadin contains vanadium(IV). Initially, amavadin was thought to have a vanadyl, VO2+, center. In 1993, it was discovered by crystallographic characterization that amavadin is not a vanadyl ion compound. Instead, it is an octacoordinated vanadium(IV) complex. This complex is bonded to two tetradentate ligands derived from N-hydroxyimino-2,2'-dipropionic acid, H3(HIDPA), ligands.[5] The ligands coordinate through the nitrogen and the three oxygen centers.
Amavadin is a C2-symmetric anion with a 2− charge. The twofold axis bisects the vanadium atom perpendicular to the two NO ligands. The anion features five chiral centers, one at vanadium and the four carbon atoms having S stereochemistry.[1] There are two possible diastereomers for the ligands, (S,S)-(S,S)-Δ and (S,S)-(S,S)-Λ.
Biological function
The biological function of amavadin is still unknown, yet it has been thought that it uses H2O2 and acts as a peroxidase to aid the regeneration of damaged tissues.[3] Amavadin may serve as a toxin for protection of the mushroom.[6]
References
- Berry, R.E.; Armstrong, E.M.; Beddoes, R.L.; Collison, D.; Ertok, S.N.; Helliwell, M.; Garner, C.D. (1999). "The Structural Characterization of Amavadin". Angew. Chem. Int. Ed. 38 (6): 795–797. doi:10.1002/(SICI)1521-3773(19990315)38:6<795::AID-ANIE795>3.0.CO;2-7. PMID 29711812.
- Kneifel, H.; Bayer, E. “Stereochemistry and total synthesis of amavadin, the naturally occurring vanadium anion of Amanita muscaria.” J. Am. Chem. Soc. 1986, 108:11, pp. 3075–3077. Kneifel, H.; Bayer, E. (1986). "Stereochemistry and total synthesis of amavadin, the naturally occurring vanadium compound of Amanita muscaria". Journal of the American Chemical Society. 108 (11): 3075–3077. doi:10.1021/ja00271a043..
- Hubregtse, T.; Neeleman, E.; Maschmeyer, T.; Sheldon, R.A.; Hanefeld, U.; Arends, I.W.C.E. (2005). "The first enantioselective synthesis of the amavadin ligand and its complexation to vanadium". J. Inorg. Biochem. 99 (5): 1264–1267. doi:10.1016/j.jinorgbio.2005.02.004. PMID 15833352.
- Fu, S-C.J.; Birnbaum, S.M.; Greenstein, J.P. (1954). "Influence of Optically Active Acyl Groups on the Enzymatic Hydrolysis of N-Acylated-L-amino Acids". J. Am. Chem. Soc. 76 (23): 6054–6058. doi:10.1021/ja01652a057.
- Armstrong, E.M.; Beddoes, R.L.; Calviou, L.J.; Charnock, J.M.; Collison, D.; Ertok, N.; Naismith, J.H.; Garner, C.D. (1993). "The Chemical Nature of Amavadin". J. Am. Chem. Soc. 115 (2): 807–808. doi:10.1021/ja00055a073.
- Garner, C.D.; Armstrong, E.M.; Berry, R.E.; Beddoes, R.L.; Collison, D.; Cooney, J.J.A.; Ertok, S.N.; Helliwell, M. (2000). "Investigations of Amavadin". J. Inorg. Biochem. 80 (1–2): 17–20. doi:10.1016/S0162-0134(00)00034-9. PMID 10885458.
