Ununennium
Ununennium, also known as eka-francium or element 119, is the hypothetical chemical element with symbol Uue and atomic number 119. Ununennium and Uue are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be an s-block element, an alkali metal, and the first element in the eighth period. It is the lightest element that has not yet been synthesized.
| Ununennium | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˌuːn.uːnˈɛniəm/ ( | ||||||||||||||||||||
| Alternative names | element 119, eka-francium | ||||||||||||||||||||
| Mass number | [315] (predicted) | ||||||||||||||||||||
| Ununennium in the periodic table | |||||||||||||||||||||
| |||||||||||||||||||||
| Atomic number (Z) | 119 | ||||||||||||||||||||
| Group | group 1: H and alkali metals | ||||||||||||||||||||
| Period | period 8 | ||||||||||||||||||||
| Block | s-block | ||||||||||||||||||||
| Element category | Unknown chemical properties, but predicted to be an alkali metal | ||||||||||||||||||||
| Electron configuration | [Og] 8s1 (predicted)[1] | ||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 18, 8, 1 (predicted) | ||||||||||||||||||||
| Physical properties | |||||||||||||||||||||
| Phase at STP | unknown (could be solid or liquid)[1] | ||||||||||||||||||||
| Melting point | 273–303 K (0–30 °C, 32–86 °F) (predicted)[1] | ||||||||||||||||||||
| Boiling point | 903 K (630 °C, 1166 °F) (predicted)[2] | ||||||||||||||||||||
| Density (near r.t.) | 3 g/cm3 (predicted)[1] | ||||||||||||||||||||
| Heat of fusion | 2.01–2.05 kJ/mol (extrapolated)[3] | ||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||
| Oxidation states | (+1), (+3) (predicted)[1] | ||||||||||||||||||||
| Electronegativity | Pauling scale: 0.86 (predicted)[4] | ||||||||||||||||||||
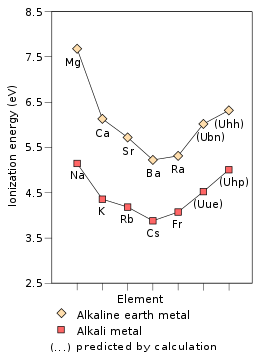
| Ionization energies |
| ||||||||||||||||||||
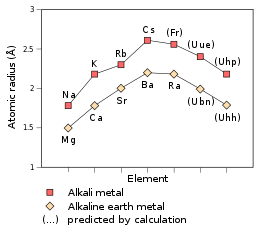
| Atomic radius | empirical: 240 pm (predicted)[1] | ||||||||||||||||||||
| Covalent radius | 263–281 pm (extrapolated)[3] | ||||||||||||||||||||
| Other properties | |||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc) (extrapolated)[6] | ||||||||||||||||||||
| CAS Number | 54846-86-5 | ||||||||||||||||||||
| History | |||||||||||||||||||||
| Naming | IUPAC systematic element name | ||||||||||||||||||||
| Main isotopes of ununennium | |||||||||||||||||||||
| |||||||||||||||||||||
Experiments aimed at the synthesis of ununennium began in June 2018 at RIKEN in Japan; another attempt by the team at the Joint Institute for Nuclear Research at Dubna, Russia is planned to begin in late 2020. Prior to this, two unsuccessful attempts had been made to synthesize ununennium, one by an American team and one by a German team. Theoretical and experimental evidence has shown that the synthesis of ununennium will likely be far more difficult than that of the previous elements, and it may even be the penultimate element that can be synthesized with current technology.
Ununennium's position as the seventh alkali metal suggests that it would have similar properties to its lighter congeners: lithium, sodium, potassium, rubidium, caesium, and francium. However, relativistic effects may cause some of its properties to differ from those expected from a straight application of periodic trends. For example, ununennium is expected to be less reactive than caesium and francium and to be closer in behavior to potassium or rubidium, and while it should show the characteristic +1 oxidation state of the alkali metals, it is also predicted to show the +3 oxidation state, which is unknown in any other alkali metal.
History
Superheavy elements are produced by nuclear fusion. These fusion reactions can be divided into "hot" and "cold" fusion,[lower-alpha 1] depending on the excitation energy of the compound nucleus produced. In hot fusion reactions, very light, high-energy projectiles are accelerated toward very heavy targets (actinides), giving rise to compound nuclei at high excitation energy (~40–50 MeV) that may fission, or alternatively evaporate several (3 to 5) neutrons.[9] In cold fusion reactions (which use heavier projectiles, typically from the fourth period, and lighter targets, usually lead and bismuth), the fused nuclei produced have a relatively low excitation energy (~10–20 MeV), which decreases the probability that these products will undergo fission reactions. As the fused nuclei cool to the ground state, they require emission of only one or two neutrons. However, hot fusion reactions tend to produce more neutron-rich products because the actinides have the highest neutron-to-proton ratios of any elements that can presently be made in macroscopic quantities.[10]
Ununennium and unbinilium (elements 119 and 120) are the elements with the lowest atomic numbers that have not yet been synthesized. Attempts to synthesize them would push the limits of current technology, due to the decreasing cross sections of the production reactions and their probably short half-lives,[11] expected to be on the order of microseconds.[1][7] Elements beyond unbiunium (element 121) would likely be too short-lived to be detected with current technology: they would decay within a microsecond, before reaching the detectors. The possibility of detection of elements 121 through 124 depends greatly on the theoretical model being used, as their half-lives are predicted to be very close to the one-microsecond border.[11] Previously, important help (characterized as "silver bullets") in the synthesis of superheavy elements came from the deformed nuclear shells around hassium-270 which increased the stability of surrounding nuclei, and the existence of the quasi-stable neutron-rich isotope calcium-48 which could be used as a projectile to produce more neutron-rich isotopes of superheavy elements.[12] The more neutron-rich a superheavy nuclide is, the closer it is expected to be to the sought-after island of stability.[lower-alpha 2] Even so, the synthesized isotopes still have fewer neutrons than those expected to be in the island of stability.[15] Furthermore, using calcium-48 to synthesize ununennium would require a target of einsteinium-253 or -254, which are very difficult to produce in sufficiently large quantities (only micrograms are presently available; in comparison, milligrams of berkelium and californium are available). More practical production of further superheavy elements would require projectiles heavier than 48Ca.[12]
Synthesis attempts
Past
The synthesis of ununennium was first attempted in 1985 by bombarding a target of einsteinium-254 with calcium-48 ions at the superHILAC accelerator at Berkeley, California:
- 254
99Es
+ 48
20Ca
→ 302
119Uue
* → no atoms
No atoms were identified, leading to a limiting cross section of 300 nb.[16] Later calculations suggest that the cross section of the 3n reaction (which would result in 299Uue and three neutrons as products) would actually be six hundred thousand times lower than this upper bound, at 0.5 pb.[17]
As ununennium is the lightest undiscovered element, it has been the target of synthesis experiments by German, Russian, and Japanese teams in recent years. The Russian team at the Joint Institute for Nuclear Research in Dubna, Russia planned to conduct an experiment before 2012, and no results were released, strongly implying that either the experiment was not done or no ununennium atoms were identified. From April to September 2012, an attempt to synthesize the isotopes 295Uue and 296Uue was made by bombarding a target of berkelium-249 with titanium-50 at the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany.[18][19] Based on the theoretically predicted cross section, it was expected that an ununennium atom would be synthesized within five months of the beginning of the experiment.[11]
- 249
97Bk
+ 50
22Ti
→ 299
119Uue
* → 296
119Uue
+ 3 1
0
n - 249
97Bk
+ 50
22Ti
→ 299
119Uue
* → 295
119Uue
+ 4 1
0
n
The experiment was originally planned to continue to November 2012,[20] but was stopped early to make use of the 249Bk target to confirm the synthesis of tennessine (thus changing the projectiles to 48Ca).[21] This reaction between 249Bk and 50Ti was predicted to be the most favorable practical reaction for formation of ununennium,[19] as it is rather asymmetrical,[11] though also somewhat cold.[21] (The reaction between 254Es and 48Ca would be superior, but preparing milligram quantities of 254Es for a target is difficult.)[11] Nevertheless, the necessary change from the "silver bullet" 48Ca to 50Ti divides the expected yield of ununennium by about twenty, as the yield is strongly dependent on the asymmetry of the fusion reaction.[11]
Due to the predicted short half-lives, the GSI team used new "fast" electronics capable of registering decay events within microseconds.[19] No ununennium atoms were identified, implying a limiting cross section of 70 fb.[21] The predicted actual cross section is around 40 fb, which is at the limits of current technology.[11] (The record lowest cross section of an experimentally successful reaction is 30 fb for the reaction between 209Bi and 70Zn producing nihonium.)[11]
Present
The team at RIKEN in Wakō, Japan began bombarding curium-248 targets with a vanadium-51 beam in June 2018[22] to search for element 119. Curium was chosen as a target, rather than heavier berkelium or californium, as these heavier targets are difficult to prepare.[23] The 248Cm targets were provided by Oak Ridge National Laboratory, which had provided the necessary 249Bk target from the synthesis of tennessine (element 117) at Dubna. The RIKEN experiment began by being conducted at a cyclotron while it upgrades its linear accelerators, which resume operation in 2020. Bombardment may be continued with both machines until the first event is observed; the experiment is currently running intermittently for at least 100 days per year.[23][24] Hideto En'yo, director of the RIKEN Nishina Centre, predicted that elements 119 and 120 would probably be discovered by 2022.[25] The RIKEN team's efforts are being bankrolled by the Emperor of Japan.[26]
- 248
96Cm
+ 51
23V
→ 299
119Uue
* → 296
119Uue
+ 3 1
0
n - 248
96Cm
+ 51
23V
→ 299
119Uue
* → 295
119Uue
+ 4 1
0
n
The produced isotopes of ununennium are expected to undergo two alpha decays to known isotopes of moscovium (288Mc and 287Mc respectively), which would anchor them to a known sequence of five further alpha decays and corroborate their production. The predicted cross section for these reactions is about 10 fb.[23]
Planned
Following the claimed synthesis of 293Og in 1999 at the Lawrence Berkeley National Laboratory from 208Pb and 86Kr, the analogous reactions 209Bi + 86Kr and 208Pb + 87Rb were proposed for the synthesis of element 119 and its then-unknown alpha decay daughters, elements 117, 115, and 113.[27] The retraction of these results in 2001[28] and more recent calculations on the cross sections for "cold" fusion reactions cast doubt on this possibility; for example, a maximum yield of 2 fb is predicted for the production of 294Uue in the former reaction.[29] Radioactive ion beams may provide an alternative method utilizing a lead or bismuth target, and may enable the production of more neutron-rich isotopes should they become available at required intensities.[29]
The team at the Joint Institute for Nuclear Research in Dubna, Russia planned to begin new experiments on the synthesis of ununennium using the 249Bk + 50Ti reaction in 2019 using a new experimental complex.[30][31][32][33][34][35] As of November 2019, the plan was for the experiment to begin in late 2020 and last approximately 150 days, with results in mid-2021 at the earliest.[36]
The laboratories at RIKEN in Japan and at the JINR in Russia are best suited to these experiments as they are the only ones in the world where long beam times are accessible for reactions with such low predicted cross sections.[37]
Naming
Using Mendeleev's nomenclature for unnamed and undiscovered elements, ununennium should be known as eka-francium. Using the 1979 IUPAC recommendations, the element should be temporarily called ununennium (symbol Uue) until it is discovered, the discovery is confirmed, and a permanent name chosen.[38] Although widely used in the chemical community on all levels, from chemistry classrooms to advanced textbooks, the recommendations are mostly ignored among scientists who work theoretically or experimentally on superheavy elements, who call it "element 119", with the symbol E119, (119) or 119.[1]
Predicted properties
Nuclear stability and isotopes
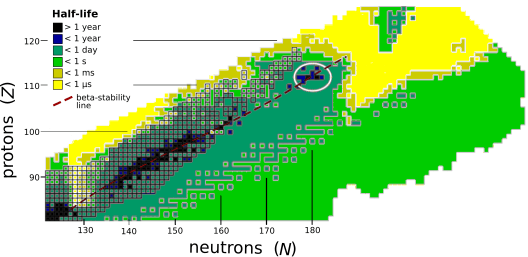
The stability of nuclei decreases greatly with the increase in atomic number after curium, element 96, whose half-life is four orders of magnitude longer than that of any currently known higher-numbered element. All isotopes with an atomic number above 101 undergo radioactive decay with half-lives of less than 30 hours. No elements with atomic numbers above 82 (after lead) have stable isotopes.[40] Nevertheless, for reasons not yet well understood, there is a slight increase of nuclear stability around atomic numbers 110–114, which leads to the appearance of what is known in nuclear physics as the "island of stability". This concept, proposed by University of California professor Glenn Seaborg, explains why superheavy elements last longer than predicted.[41]
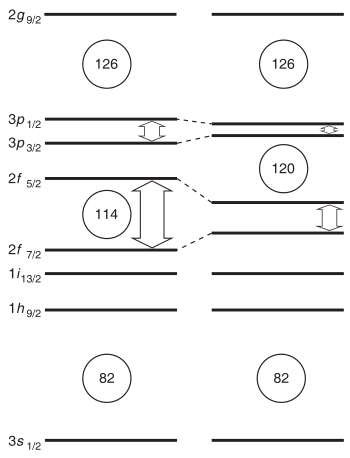
The alpha-decay half-lives predicted for 291–307Uue are on the order of microseconds. The longest alpha-decay half-life predicted is ~485 microseconds for the isotope 294Uue.[42][43][44] When factoring in all decay modes, the predicted half-lives drop further to only tens of microseconds.[1][7] Some heavier isotopes may be more stable; Fricke and Waber predicted 315Uue to be the most stable ununennium isotope in 1971.[2] This has consequences for the synthesis of ununennium, as isotopes with half-lives below one microsecond would decay before reaching the detector, and the heavier isotopes cannot be synthesised by the collision of any known usable target and projectile nuclei.[1][7] Nevertheless, new theoretical models show that the expected gap in energy between the proton orbitals 2f7/2 (filled at element 114) and 2f5/2 (filled at element 120) is smaller than expected, so that element 114 no longer appears to be a stable spherical closed nuclear shell, and this energy gap may increase the stability of elements 119 and 120. The next doubly magic nucleus is now expected to be around the spherical 306Ubb (element 122), but the expected low half-life and low production cross section of this nuclide makes its synthesis challenging.[39]
Atomic and physical
Being the first period 8 element, ununennium is predicted to be an alkali metal, taking its place in the periodic table below lithium, sodium, potassium, rubidium, caesium, and francium. Each of these elements has one valence electron in the outermost s-orbital (valence electron configuration ns1), which is easily lost in chemical reactions to form the +1 oxidation state: thus the alkali metals are very reactive elements. Ununennium is predicted to continue the trend and have a valence electron configuration of 8s1. It is therefore expected to behave much like its lighter congeners; however, it is also predicted to differ from the lighter alkali metals in some properties.[1]
The main reason for the predicted differences between ununennium and the other alkali metals is the spin–orbit (SO) interaction—the mutual interaction between the electrons' motion and spin. The SO interaction is especially strong for the superheavy elements because their electrons move faster—at velocities comparable to the speed of light—than those in lighter atoms.[45] In ununennium atoms, it lowers the 7p and 8s electron energy levels, stabilizing the corresponding electrons, but two of the 7p electron energy levels are more stabilized than the other four.[46] The effect is called subshell splitting, as it splits the 7p subshell into more-stabilized and the less-stabilized parts. Computational chemists understand the split as a change of the second (azimuthal) quantum number l from 1 to 1/2 and 3/2 for the more-stabilized and less-stabilized parts of the 7p subshell, respectively.[45][lower-alpha 3] Thus, the outer 8s electron of ununennium is stabilized and becomes harder to remove than expected, while the 7p3/2 electrons are correspondingly destabilized, perhaps allowing them to participate in chemical reactions.[1] This stabilization of the outermost s-orbital (already significant in francium) is the key factor affecting ununennium's chemistry, and causes all the trends for atomic and molecular properties of alkali metals to reverse direction after caesium.[4]
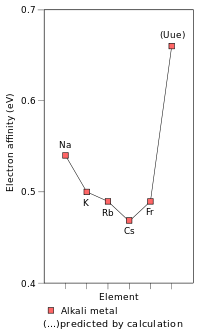 Empirical (Na–Cs), semi-empirical (Fr), and predicted (Uue) electron affinities of the alkali metals from the third to the eighth period, measured in electron volts.[1][47] They decrease from Li to Cs, but the Fr value, 492±10 meV, is 20 meV higher than that of Cs, and that of Uue is much higher still at 662 meV.[48] |
Due to the stabilization of its outer 8s electron, ununennium's first ionization energy—the energy required to remove an electron from a neutral atom—is predicted to be 4.53 eV, higher than those of the known alkali metals from potassium onward. This effect is so large that unbiunium (element 121) is predicted to have a lower ionization energy of 4.45 eV, so that the alkali metal in period 8 would not have the lowest ionization energy in the period, as is true for all previous periods.[1] Ununennium's electron affinity is expected to be far greater than that of caesium and francium; indeed, ununennium is expected to have an electron affinity higher than all the alkali metals lighter than it at about 0.662 eV, close to that of cobalt (0.662 eV) and chromium (0.676 eV).[48] Relativistic effects also cause a very large drop in the polarizability of ununennium[1] to 169.7 a.u.[49] Indeed, the static dipole polarisability (αD) of ununennium, a quantity for which the impacts of relativity are proportional to the square of the element's atomic number, has been calculated to be small and similar to that of sodium.[50]
The electron of the hydrogen-like ununennium atom—oxidized so it has only one electron, Uue118+—is predicted to move so quickly that its mass is 1.99 times that of a non-moving electron, a feature coming from the relativistic effects. For comparison, the figure for hydrogen-like francium is 1.29 and the figure for hydrogen-like caesium is 1.091.[45] According to simple extrapolations of relativity laws, that indirectly indicates the contraction of the atomic radius[45] to around 240 pm,[1] very close to that of rubidium (247 pm); the metallic radius is also correspondingly lowered to 260 pm.[1] The ionic radius of Uue+ is expected to be 180 pm.[1]
Ununennium is predicted to have a melting point between 0 °C and 30 °C: thus it may be a liquid at room temperature.[5] It is not known whether this continues the trend of decreasing melting points down the group, as caesium's melting point is 28.5 °C and francium's is estimated to be around 8.0 °C.[51] The boiling point of ununennium is expected to be around 630 °C, similar to that of francium, estimated to be around 620 °C; this is lower than caesium's boiling point of 671 °C.[2][51] The density of ununennium has been variously predicted to be between 3 and 4 g/cm3, continuing the trend of increasing density down the group: the density of francium is estimated at about 2.48 g/cm3, and that of caesium is known to be 1.93 g/cm3.[2][3][51]
Chemical
Bond lengths and bond-dissociation energies of alkali metal dimers. Data for Fr2 and Uue2 is predicted.[52] Compound Bond length (Å) Bond-dissociation energy (kJ/mol) Li2 2.673 101.9 Na2 3.079 72.04 K2 3.924 53.25 Rb2 4.210 47.77 Cs2 4.648 43.66 Fr2 ~ 4.61 ~ 42.1 Uue2 ~ 4.27 ~ 53.4
The chemistry of ununennium is predicted to be similar to that of the alkali metals,[1] but it would probably behave more like potassium[53] or rubidium[1] than caesium or francium. This is due to relativistic effects, as in their absence periodic trends would predict ununennium to be even more reactive than caesium and francium. This lowered reactivity is due to the relativistic stabilization of ununennium's valence electron, increasing ununennium's first ionization energy and decreasing the metallic and ionic radii;[53] this effect is already seen for francium.[1]
The chemistry of ununennium in the +1 oxidation state should be more similar to the chemistry of rubidium than to that of francium. On the other hand, the ionic radius of the Uue+ ion is predicted to be larger than that of Rb+, because the 7p orbitals are destabilized and are thus larger than the p-orbitals of the lower shells. Ununennium may also show the +3 oxidation state,[1] which is not seen in any other alkali metal,[54] in addition to the +1 oxidation state that is characteristic of the other alkali metals and is also the main oxidation state of all the known alkali metals: this is because of the destabilization and expansion of the 7p3/2 spinor, causing its outermost electrons to have a lower ionization energy than what would otherwise be expected.[1][54] Many ununennium compounds are expected to have a large covalent character, due to the involvement of the 7p3/2 electrons in the bonding: this effect is also seen to a lesser extent in francium, which shows some 6p3/2 contribution to the bonding in francium superoxide (FrO2).[45] Thus, instead of ununennium being the most electropositive element, as a simple extrapolation would seem to indicate, caesium instead retains this position, with ununennium's electronegativity most likely being close to sodium's (0.93 on the Pauling scale).[4] The standard reduction potential of the Uue+/Uue couple is predicted to be −2.9 V, the same as that of the Fr+/Fr couple and just over that of the K+/K couple at −2.931 V.[5]
Bond lengths and bond-dissociation energies of MAu (M = an alkali metal). All data is predicted, except for the bond-dissociation energies of KAu, RbAu, and CsAu.[4] Compound Bond length (Å) Bond-dissociation energy (kJ/mol) KAu 2.856 2.75 RbAu 2.967 2.48 CsAu 3.050 2.53 FrAu 3.097 2.75 UueAu 3.074 2.44
In the gas phase, and at very low temperatures in the condensed phase, the alkali metals form covalently bonded diatomic molecules. The metal–metal bond lengths in these M2 molecules increase down the group from Li2 to Cs2, but then decrease after that to Uue2, due to the aforementioned relativistic effects that stabilize the 8s orbital. The opposite trend is shown for the metal–metal bond-dissociation energies. The Uue–Uue bond should be slightly stronger than the K–K bond.[4][52] From these M2 dissociation energies, the enthalpy of sublimation (ΔHsub) of ununennium is predicted to be 94 kJ/mol (the value for francium should be around 77 kJ/mol).[4]
The UueF molecule is expected to have a significant covalent character owing to the high electron affinity of ununennium. The bonding in UueF is predominantly between a 7p orbital on ununennium and a 2p orbital on fluorine, with lesser contributions from the 2s orbital of fluorine and the 8s, 6dz2, and the two other 7p orbitals of ununennium. This is very different from the behaviour of s-block elements, as well as gold and mercury, in which the s-orbitals (sometimes mixed with d-orbitals) are the ones participating in the bonding. The Uue–F bond is relativistically expanded due to the splitting of the 7p orbital into 7p1/2 and 7p3/2 spinors, forcing the bonding electrons into the largest orbital measured by radial extent: a similar expansion in bond length is found in the hydrides AtH and TsH.[55] The Uue–Au bond should be the weakest of all bonds between gold and an alkali metal, but should still be stable. This gives extrapolated medium-sized adsorption enthalpies (−ΔHads) of 106 kJ/mol on gold (the francium value should be 136 kJ/mol), 76 kJ/mol on platinum, and 63 kJ/mol on silver, the smallest of all the alkali metals, that demonstrate that it would be feasible to study the chromatographic adsorption of ununennium onto surfaces made of noble metals.[4] The enthalpy of adsorption of ununennium on a Teflon surface is predicted to be 17.6 kJ/mol, which would be the lowest among the alkali metals: this information would be very useful for future chemistry experiments on ununennium.[49] The ΔHsub and −ΔHads values are not proportionally related for the alkali metals, as they change in opposite directions as atomic number increases.[4]
See also
Notes
- Despite the name, "cold fusion" in the context of superheavy element synthesis is a distinct concept from the idea that nuclear fusion can be achieved in room temperature conditions (see cold fusion).[8]
- Stable isotopes of the lightest elements usually have a neutron–proton ratio close or equal to one (for example, the only stable isotope of aluminium has 13 protons and 14 neutrons,[13] making a neutron–proton ratio of 1.077). However, isotopes of heavier elements have higher neutron–proton ratios, increasing with the number of protons (iodine's only stable isotope has 53 protons and 74 neutrons, neutron–proton ratio of 1.396; gold's only stable isotope has 79 protons and 118 neutrons, neutron–proton ratio of 1.494; plutonium's most stable isotope has 94 protons and 150 neutrons, neutron–proton ratio of 1.596).[13] The trend is expected to continue to the superheavy elements,[14] making it difficult to synthesize their most stable isotopes, because the neutron–proton ratios of the elements they are synthesized from are lower than the expected ratios of the most stable isotopes of the superheavy elements.
- The quantum number corresponds to the letter in the electron orbital name: 0 to s, 1 to p, 2 to d, etc. See azimuthal quantum number for more information.
References
- Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN 978-1-4020-3555-5.
- Fricke, B.; Waber, J. T. (1971). "Theoretical Predictions of the Chemistry of Superheavy Elements" (PDF). Actinides Reviews. 1: 433–485. Retrieved 7 August 2013.
- Bonchev, Danail; Kamenska, Verginia (1981). "Predicting the Properties of the 113–120 Transactinide Elements". Journal of Physical Chemistry. American Chemical Society. 85 (9): 1177–1186. doi:10.1021/j150609a021.
- Pershina, V.; Borschevsky, A.; Anton, J. (20 February 2012). "Fully relativistic study of intermetallic dimers of group-1 elements K through element 119 and prediction of their adsorption on noble metal surfaces". Chemical Physics. Elsevier. 395: 87–94. Bibcode:2012CP....395...87P. doi:10.1016/j.chemphys.2011.04.017. This article gives the Mulliken electronegativity as 2.862, which has been converted to the Pauling scale via χP = 1.35χM1/2 − 1.37.
- Fricke, Burkhard (1975). "Superheavy elements: a prediction of their chemical and physical properties". Recent Impact of Physics on Inorganic Chemistry. 21: 89–144. doi:10.1007/BFb0116498. Retrieved 4 October 2013.
- Seaborg, Glenn T. (1969). "Prospects for further considerable extension of the periodic table" (PDF). Journal of Chemical Education. 46 (10): 626–634. Bibcode:1969JChEd..46..626S. doi:10.1021/ed046p626. Retrieved 22 February 2018.
- Hofmann, Sigurd (2013). Overview and Perspectives of SHE Research at GSI SHIP. p. 23–32. doi:10.1007/978-3-319-00047-3.
- Fleischmann, Martin; Pons, Stanley (1989). "Electrochemically induced nuclear fusion of deuterium". Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 261 (2): 301–308. doi:10.1016/0022-0728(89)80006-3.
- Barber, Robert C.; Gäggeler, Heinz W.; Karol, Paul J.; Nakahara, Hiromichi; Vardaci, Emanuele; Vogt, Erich (2009). "Discovery of the element with atomic number 112 (IUPAC Technical Report)". Pure and Applied Chemistry. 81 (7): 1331. doi:10.1351/PAC-REP-08-03-05.
- Armbruster, Peter & Munzenberg, Gottfried (1989). "Creating superheavy elements". Scientific American. 34: 36–42.
- Zagrebaev, Valeriy; Karpov, Alexander; Greiner, Walter (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?" (PDF). Journal of Physics. 420: 012001. arXiv:1207.5700. Bibcode:2013JPhCS.420a2001Z. doi:10.1088/1742-6596/420/1/012001.
- Folden III, C. M.; Mayorov, D. A.; Werke, T. A.; Alfonso, M. C.; Bennett, M. E.; DeVanzo, M. J. (2013). "Prospects for the discovery of the next new element: Influence of projectiles with Z > 20". Journal of Physics: Conference Series. 420 (1): 012007. arXiv:1209.0498. Bibcode:2013JPhCS.420a2007F. doi:10.1088/1742-6596/420/1/012007.
- Audi, Georges; Bersillon, Olivier; Blachot, Jean; et al. (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729 (1): 3–128. Bibcode:2003NuPhA.729....3A. CiteSeerX 10.1.1.692.8504. doi:10.1016/j.nuclphysa.2003.11.001. Archived from the original (PDF) on 2011-07-20. Retrieved 2010-07-05.
- Karpov, A. V.; Zagrebaev, V. I.; Palenzuela, Y. Martinez; Greiner, Walter (2013). "Superheavy Nuclei: Decay and Stability". Exciting Interdisciplinary Physics. p. 69. doi:10.1007/978-3-319-00047-3_6. ISBN 978-3-319-00046-6.
- "Universal nuclide chart". Nucleonica. Institute for Transuranium Elements. 2007–2012. Retrieved 2012-07-03. (registration required)
- Lougheed, R.; Landrum, J.; Hulet, E.; Wild, J.; Dougan, R.; Dougan, A.; Gäggeler, H.; Schädel, M.; Moody, K. (1985). "Search for superheavy elements using 48Ca + 254Esg reaction". Physical Review C. 32 (5): 1760–1763. Bibcode:1985PhRvC..32.1760L. doi:10.1103/PhysRevC.32.1760. PMID 9953034.
- Feng, Z; Jin, G.; Li, J.; Scheid, W. (2009). "Production of heavy and superheavy nuclei in massive fusion reactions". Nuclear Physics A. 816 (1): 33. arXiv:0803.1117. Bibcode:2009NuPhA.816...33F. doi:10.1016/j.nuclphysa.2008.11.003.
- Modern alchemy: Turning a line, The Economist, May 12, 2012.
- Superheavy Element Search Campaign at TASCA. J. Khuyagbaatar
- "Search for element 119: Christoph E. Düllmann for the TASCA E119 collaboration" (PDF). Archived from the original (PDF) on 2016-03-04. Retrieved 2015-09-15.
- Superheavy Element Research at TASCA. Alexander Yakushev
- Ball, P. (2019). "Extreme chemistry: experiments at the edge of the periodic table". Nature. 565 (7741): 552–555. Bibcode:2019Natur.565..552B. doi:10.1038/d41586-019-00285-9. ISSN 1476-4687. PMID 30700884.
- Sakai, Hideyuki (27 February 2019). "Search for a New Element at RIKEN Nishina Center" (PDF). infn.it. Retrieved 17 December 2019.
- Ball, P. (2019). "Extreme chemistry: experiments at the edge of the periodic table" (PDF). Nature. 565 (7741): 552–555. Bibcode:2019Natur.565..552B. doi:10.1038/d41586-019-00285-9. PMID 30700884.
- "Hunt for element 119 set to begin". Chemistry World. 12 September 2017. Retrieved 9 January 2018.
- Chapman, Kit; Turner, Kristy (13 February 2018). "The hunt is on". Education in Chemistry. Royal Society of Chemistry. Retrieved 28 June 2019.
The hunt for element 113 was almost abandoned because of lack of resources, but this time Japan’s emperor is bankrolling Riken’s efforts to extend the periodic table to its eighth row.
- Hoffman, D.C; Ghiorso, A.; Seaborg, G.T. (2000). The Transuranium People: The Inside Story. Imperial College Press. ISBN 978-1-86094-087-3.
- Public Affairs Department (21 July 2001). "Results of element 118 experiment retracted". Berkeley Lab. Archived from the original on 29 January 2008. Retrieved 18 January 2008.
- Loveland, W. (2007). "Synthesis of transactinide nuclei using radioactive beams" (PDF). Physical Review C. 76 (1): 014612. Bibcode:2007PhRvC..76a4612L. doi:10.1103/PhysRevC.76.014612.
- "Scientists will begin experiments on the synthesis of element 119 in 2019". www.jinr.ru. JINR. 28 September 2016. Retrieved 31 March 2017.
“The discovery of elements 115, 117 and 118 is an accomplished fact; they were placed in the periodic table, though still unnamed and will be confirmed only at the end of the year. The D.I.Mendeleev Periodic Table is not infinite. In 2019, scientists will begin the synthesis of elements 119 and 120 which are the first in the 8th period,” said S.N. Dmitriev.
- Dmitriev, Sergey; Itkis, Mikhail; Oganessian, Yuri (2016). Status and perspectives of the Dubna superheavy element factory (PDF). Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements. doi:10.1051/epjconf/201613108001.
- "What it takes to make a new element". Chemistry World. Retrieved 2016-12-03.
- Roberto, J. B. (31 March 2015). "Actinide Targets for Super-Heavy Element Research" (PDF). cyclotron.tamu.edu. Texas A & M University. Retrieved 28 April 2017.
- Morita, Kōsuke (5 February 2016). "The Discovery of Element 113". YouTube. Retrieved 28 April 2017.
- Morimoto, Kouji (2016). "The discovery of element 113 at RIKEN" (PDF). www.physics.adelaide.edu.au. 26th International Nuclear Physics Conference. Retrieved 14 May 2017.
-
"Yu. Oganessian commented on synthesis of new elements". JINR. 18 November 2019. Retrieved 16 June 2020.
Academician notes that the target will be completed until the end of 2020, and another 150 days will be spent on experiments with the target for the synthesis of element 119. Thus, results should be awaited not earlier than the middle of 2021.
- Hagino, Kouichi; Hofmann, Sigurd; Miyatake, Hiroari; Nakahara, Hiromichi (2012). "平成23年度 研究業績レビュー(中間レビュー)の実施について" (PDF). www.riken.jp. RIKEN. Archived from the original (PDF) on 4 November 2018. Retrieved 5 May 2017.
- Chatt, J. (1979). "Recommendations for the naming of elements of atomic numbers greater than 100". Pure and Applied Chemistry. 51 (2): 381–384. doi:10.1351/pac197951020381.
- Kratz, J. V. (5 September 2011). The Impact of Superheavy Elements on the Chemical and Physical Sciences (PDF). 4th International Conference on the Chemistry and Physics of the Transactinide Elements. Retrieved 27 August 2013.
- de Marcillac, Pierre; Coron, Noël; Dambier, Gérard; et al. (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201.
- Considine, Glenn D.; Kulik, Peter H. (2002). Van Nostrand's scientific encyclopedia (9th ed.). Wiley-Interscience. ISBN 978-0-471-33230-5. OCLC 223349096.
- Chowdhury, P. Roy; Samanta, C. & Basu, D. N. (2007). "Predictions of alpha decay half lives of heavy and superheavy elements". Nucl. Phys. A. 789 (1–4): 142–154. arXiv:nucl-th/0703086. Bibcode:2007NuPhA.789..142S. CiteSeerX 10.1.1.264.8177. doi:10.1016/j.nuclphysa.2007.04.001.
- Chowdhury, P. Roy; Samanta, C. & Basu, D. N. (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Phys. Rev. C. 77 (4): 044603. arXiv:0802.3837. Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603.
- Chowdhury, P. Roy; Samanta, C. & Basu, D. N. (2008). "Nuclear half-lives for α -radioactivity of elements with 100 ≤ Z ≤ 130". Atomic Data and Nuclear Data Tables. 94 (6): 781–806. arXiv:0802.4161. Bibcode:2008ADNDT..94..781C. doi:10.1016/j.adt.2008.01.003.
- Thayer, John S. (2010). "Relativistic Effects and the Chemistry of the Heavier Main Group Elements". In Maria, Barysz; Ishikawa, Yasuyuki (eds.). Relativistic Methods for Chemists. Challenges and Advances in Computational Chemistry and Physics. 10. Springer Netherlands. pp. 63–7, 81, 84. doi:10.1007/978-1-4020-9975-5_2. ISBN 978-1-4020-9974-8.
- Fægri Jr., Knut; Saue, Trond (2001). "Diatomic molecules between very heavy elements of group 13 and group 17: A study of relativistic effects on bonding". The Journal of Chemical Physics. 115 (6): 2456. Bibcode:2001JChPh.115.2456F. doi:10.1063/1.1385366.
- Pyykkö, Pekka (2011). "A suggested periodic table up to Z ≤ 172, based on Dirac–Fock calculations on atoms and ions". Physical Chemistry Chemical Physics. 13 (1): 161–8. Bibcode:2011PCCP...13..161P. doi:10.1039/c0cp01575j. PMID 20967377.
- Landau, Arie; Eliav, Ephraim; Ishikawa, Yasuyuki; Kador, Uzi (25 May 2001). "Benchmark calculations of electron affinities of the alkali atoms sodium to eka-francium (element 119)". Journal of Chemical Physics. 115 (6): 2389–92. Bibcode:2001JChPh.115.2389L. doi:10.1063/1.1386413. Retrieved 15 September 2015.
- Borschevsky, A.; Pershina, V.; Eliav, E.; Kaldor, U. (22 March 2013). "Ab initio studies of atomic properties and experimental behavior of element 119 and its lighter homologs" (PDF). The Journal of Chemical Physics. 138 (12): 124302. Bibcode:2013JChPh.138l4302B. doi:10.1063/1.4795433. PMID 23556718.
- Lim, Ivan S.; Pernpointner, Markus; Seth, Michael; Laerdahl, Jon K.; Schwerdtfeger, Peter; Neogrady, Pavel; Urban, Miroslav (1 October 1999). "Relativistic coupled-cluster static dipole polarizabilities of the alkali metals from Li to element 119". Physical Review A. 60 (4): 2822. Bibcode:1999PhRvA..60.2822L. doi:10.1103/PhysRevA.60.2822.
- Lavrukhina, Avgusta Konstantinovna; Pozdnyakov, Aleksandr Aleksandrovich (1970). Analytical Chemistry of Technetium, Promethium, Astatine, and Francium. Translated by R. Kondor. Ann Arbor–Humphrey Science Publishers. p. 269. ISBN 978-0-250-39923-9.
- Jones, Cameron; Mountford, Philip; Stasch, Andreas; Blake, Matthew P. (22 June 2015). "s-block Metal-Metal Bonds". In Liddle, Stephen T. (ed.). Molecular Metal-Metal Bonds: Compounds, Synthesis, Properties. John Wiley and Sons. pp. 23–24. ISBN 9783527335411.
- Seaborg (c. 2006). "transuranium element (chemical element)". Encyclopædia Britannica. Retrieved 2010-03-16.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- Miranda, Patrícia S.; Mendes, Anna Paula S.; Gomes, Jose S.; Alves, Claudio N.; de Souza, Aguinaldo R.; Sambrano, Julio R.; Gargano, Ricardo; de Macedo, Luiz Guilherme M. (2012). "Ab Initio Correlated All Electron Dirac-Fock Calculations for Eka-Francium Fluoride (E119F)". Journal of the Brazilian Chemical Society. 23 (6): 1104–1113. doi:10.1590/S0103-50532012000600015. Retrieved 14 January 2018.
External links