3α-Etiocholanediol
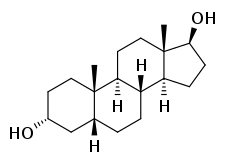
3α-Etiocholanediol, or simply etiocholanediol, also known as 3α,5β-androstanediol or as etiocholane-3α,17β-diol, is a naturally occurring etiocholane (5β-androstane) steroid and an endogenous metabolite of testosterone. It is formed from 5β-dihydrotestosterone (after 5β-reduction of testosterone) and is further transformed into etiocholanolone.[1]
 | |
| Names | |
|---|---|
| IUPAC name
(3R,5R,8R,9S,10S,13S,14S,17S)-10,13-Dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-3,17-diol | |
| Other names
Etiocholanediol; 5β-Androstane-3α,17β-diol; Etiocholane-3α,17β-diol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C19H32O2 | |
| Molar mass | 292.463 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.