3,4-Ethylenedioxythiophene
3,4-Ethylenedioxythiophene (EDOT) is the organosulfur compound with the formula C2H4O2C4H2S. The molecule consists of thiophene, substituted at the 3 and 4 positions with an ethylene glycolyl unit. It is a colorless viscous liquid.[1]
 | |
| Names | |
|---|---|
| Other names
2,3-dihydrothieno[3,4-b]-1,4-dioxin | |
| Identifiers | |
PubChem CID |
|
| Properties | |
| C6H6O2S | |
| Molar mass | 142.17 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.331 g/mL |
| Boiling point | 193 °C (379 °F; 466 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
EDOT is the precursor to the polymer PEDOT, which is found in electrochromic displays, photovoltaics, electroluminescent displays, printed wiring, and sensors.[2][3]
Synthesis and polymerization
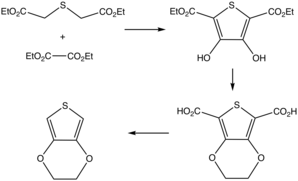
The original synthesis proceeded via the diester of 3,4-dihydroxythiophene-2,5-dicarboxylate.
EDOT is often prepared from C4 precursors such as butanediol and butadiene via routes that produce the thiophene and dioxane rings in separate steps. Representative is the reaction of 2,3-butanedione, trimethylorthoformate, and ethylene glycol to form the dioxane. Sulfidization with elemental sulfur gives the bicyclic target.[4]
EDOT is converted into the conducting polymer PEDOT by oxidation. The mechanism for this conversion begins with production of the radical cation [EDOT]+, which attacks a neutral EDOT molecule followed by deprotonation. Further similar steps result in the dehydropolymerization. The idealized conversion using peroxydisulfate is shown
- n C2H4O2C4H2S + n (OSO3)22− → [C2H4O2C4S]n + 2n HOSO3−
For commercial purposes, the polymerization is conducted in the presence of polystyrenesulfonate.[3]
References
- F. Jonas; L. Schrader. "Conductive Modifications of Polymers with Polypyrroles and Polythiophenes". Synthetic Metals. 41: 831–836. doi:10.1016/0379-6779(91)91506-6.
- Groenendaal, L. B.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J. R. (2000). "Poly(3,4-Ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future". Adv. Mater. 12: 481–494. doi:10.1002/(SICI)1521-4095(200004)12:7<481::AID-ADMA481>3.0.CO;2-C.
- Kirchmeyer, S.; Reuter, K. (2005). "Scientific Importance, Properties and Growing Applications of Poly(3,4-Ethylenedioxythiophene)". J. Mater. Chem. 15: 2077–2088. doi:10.1039/b417803n.
- Hachiya, I.; Yamamoto, T.; Inagaki, T.; et al. (2014). "Two-Step Synthesis of 3,4-Ethylenedioxythiophene (EDOT) from 2,3-Butanedione". Heterocycles. 88: 607–612. doi:10.3987/COM-13-S(S)8.