Ziegler–Natta catalyst
A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:
- Heterogeneous supported catalysts based on titanium compounds are used in polymerization reactions in combination with cocatalysts, organoaluminum compounds such as triethylaluminium, Al(C2H5)3. This class of catalyst dominates the industry.[1]
- Homogeneous catalysts usually based on complexes of Ti, Zr or Hf. They are usually used in combination with a different organoaluminum cocatalyst, methylaluminoxane (or methylalumoxane, MAO). These catalysts traditionally contains metallocenes but also feature multidentate oxygen- and nitrogen-based ligands.[2]
Ziegler–Natta catalysts are used to polymerize terminal alkenes (ethylene and alkenes with the vinyl double bond):
- n CH2=CHR → −[CH2−CHR]n−;
History
The 1963 Nobel Prize in Chemistry was awarded to German Karl Ziegler, for his discovery of first titanium-based catalysts, and Italian Giulio Natta, for using them to prepare stereoregular polymers from propylene. Ziegler–Natta catalysts have been used in the commercial manufacture of various polyolefins since 1956. As of 2010, the total volume of plastics, elastomers, and rubbers produced from alkenes with these and related (especially Phillips) catalysts worldwide exceeds 100 million tonnes. Together, these polymers represent the largest-volume commodity plastics as well as the largest-volume commodity chemicals in the world.
In the early 1950s workers at Phillips Petroleum discovered that chromium catalysts are highly effective for the low-temperature polymerization of ethylene, which launched major industrial technologies culminating in the Phillips catalyst. A few years later, Ziegler discovered that a combination of TiCl4 and Al(C2H5)2Cl gave comparable activities for the production of polyethylene. Natta used crystalline α-TiCl3 in combination with Al(C2H5)3 to produce first isotactic polypropylene.[3] Usually Ziegler catalysts refer to titanium-based systems for conversions of ethylene and Ziegler–Natta catalysts refer to systems for conversions of propylene. In the 1970s, magnesium chloride was discovered to greatly enhance the activity of the titanium-based catalysts. These catalysts were so active that the residual titanium was no longer removed from the product. They enabled the commercialization of linear low-density polyethylene (LLDPE) resins and allowed the development of noncrystalline copolymers.[4]
Also, in the 1960s, BASF developed a gas-phase, mechanically-stirred polymerization process for making polypropylene. In that process, the particle bed in the reactor was either not fluidized or not fully fluidized. In 1968, the first gas phase fluidized-bed polymerization process, the Unipol process, was commercialized by Union Carbide to produce polyethylene. In the mid-1980s, the Unipol process was further extended to produce polypropylene.
The features of the fluidized-bed process, including its simplicity and product quality, made it widely accepted all over the world. As of today, the fluidized-bed process is one of the two most widely used technologies for producing polypropylene.[5]
In the 1970s, magnesium chloride-supported Z-N catalysts were introduced. These catalysts exhibit activities so enhanced that costly steps could be omitted from the workup. These omitted processes included deashing (removal of residual catalyst) and removal of unwanted amorphous polymer.[6]
Stereochemistry of poly-1-alkenes
Natta first used polymerization catalysts based on titanium chlorides to polymerize propylene and other 1-alkenes. He discovered that these polymers are crystalline materials and ascribed their crystallinity to a special feature of the polymer structure called stereoregularity.
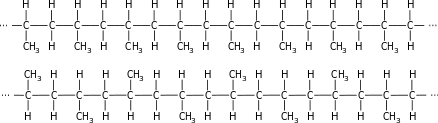
The concept of stereoregularity in polymer chains is illustrated in the picture on the left with polypropylene. Stereoregular poly(1-alkene) can be isotactic or syndiotactic depending on the relative orientation of the alkyl groups in polymer chains consisting of units −[CH2−CHR]−, like the CH3 groups in the figure. In the isotactic polymers, all stereogenic centers CHR share the same configuration. The stereogenic centers in syndiotactic polymers alternate their relative configuration. A polymer that lacks any regular arrangement in the position of its alkyl substituents (R) is called atactic. Both isotactic and syndiotactic polypropylene are crystalline, whereas atactic polypropylene, which can also be prepared with special Ziegler–Natta catalysts, is amorphous. The stereoregularity of the polymer is determined by the catalyst used to prepare it.
Classes
Heterogeneous catalysts
The first and dominant class of titanium-based catalysts (and some vanadium-based catalysts) for alkene polymerization can be roughly subdivided into two subclasses, (a) catalysts suitable for homopolymerization of ethylene and for ethylene/1-alkene copolymerization reactions leading to copolymers with a low 1-alkene content, 2–4 mol% (LLDPE resins), and (b) catalysts suitable for the synthesis of isotactic 1-alkenes. The overlap between these two subclasses is relatively small because the requirements to the respective catalysts differ widely.
Commercial catalysts are supported, i.e. bound to a solid with a high surface area. Both TiCl4 and TiCl3 give active catalysts.[7][8] The support in the majority of the catalysts is MgCl2. A third component of most catalysts is a carrier, a material that determines the size and the shape of catalyst particles. The preferred carrier is microporous spheres of amorphous silica with a diameter of 30–40 mm. During the catalyst synthesis, both the titanium compounds and MgCl2 are packed into the silica pores. All these catalysts are activated with organoaluminum compounds such as Al(C2H5)3.[8]
All modern supported Ziegler–Natta catalysts designed for polymerization of propylene and higher 1-alkenes are prepared with TiCl4 as the active ingredient and MgCl2 as a support. Another component of all such catalysts is an organic modifier, usually an ester of an aromatic diacid or a diether. The modifiers react both with inorganic ingredients of the solid catalysts as well as with organoaluminum cocatalysts.[8] These catalysts polymerize propylene and other 1-alkenes to highly crystalline isotactic polymers.[7][8]
Homogeneous catalysts
A second class of Ziegler–Natta catalysts are soluble in the reaction medium. Traditionally such homogeneous catalysts were derived from metallocenes, but the structures of active catalysts have been significantly broadened to include nitrogen-based ligands.
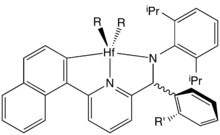
Metallocene catalysts
These catalysts are metallocenes together with a cocatalyst, typically MAO, −[O−Al(CH3)]n−. The idealized metallocene catalysts have the composition Cp2MCl2 (M = Ti, Zr, Hf) such as titanocene dichloride. Typically, the organic ligands are derivatives of cyclopentadienyl. In some complexes, the two cyclopentadiene (Cp) rings are linked with bridges, like −CH2−CH2− or >SiPh2. Depending of the type of their cyclopentadienyl ligands, for example by using an ansa-bridge, metallocene catalysts can produce either isotactic or syndiotactic polymers of propylene and other 1-alkenes.[7][8][10][11]
Non-metallocene catalysts
Ziegler–Natta catalysts of the third class, non-metallocene catalysts, use a variety of complexes of various metals, ranging from scandium to lanthanoid and actinoid metals, and a large variety of ligands containing oxygen, nitrogen, phosphorus, and sulfur. The complexes are activated using MAO, as is done for metallocene catalysts.
Most Ziegler–Natta catalysts and all the alkylaluminium cocatalysts are unstable in air, and the alkylaluminium compounds are pyrophoric. The catalysts, therefore, are always prepared and handled under an inert atmosphere.
Mechanism of Ziegler–Natta polymerization
The structure of active centers in Ziegler–Natta catalysts is well established only for metallocene catalysts. An idealized and simplified metallocene complex Cp2ZrCl2 represents a typical precatalyst. It is unreactive toward alkenes. The dihalide reacts with MAO and is transformed into a metallocenium ion Cp2Zr+CH3, which is ion-paired to some derivative(s) of MAO. A polymer molecule grows by numerous insertion reactions of C=C bonds of 1-alkene molecules into the Zr–C bond in the ion:
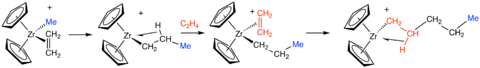
Many thousands of alkene insertion reactions occur at each active center resulting in the formation of long polymer chains attached to the center. The Cossee–Arlman mechanism describes the growth of stereospecific polymers.[3][12] This mechanism states that the polymer grows through alkene coordination at a vacant site at the titanium atom, which is followed by insertion of the C=C bond into the Ti−C bond at the active center.
Termination processes
On occasion, the polymer chain is disengaged from the active centers in the chain termination reaction. Several pathways exist for termination:
- Cp2−(CH2−CHR)n−CH3 + CH2=CHR → Cp2−CH2−CH2R + CH2=CR–polymer
Another type of chain termination reaction called β-hydrogen elimination reaction also occurs periodically:
- Cp2−(CH2−CHR)n−CH3 → Cp2−H + CH2=CR–polymer
Polymerization reactions of alkene with solid titanium-based catalysts occur at special titanium centers located on the exterior of the catalyst crystallites. Some titanium atoms in these crystallites react with organoaluminum cocatalysts with the formation of Ti–C bonds. The polymerization reaction of alkenes occurs similarly to the reactions in metallocene catalysts:
- LnTi–CH2−CHR–polymer + CH2=CHR → LnTi–CH2-CHR–CH2−CHR–polymer
The two chain termination reactions occurs quite rarely in Ziegler–Natta catalysis and the formed polymers have a too high molecular weight to be of commercial use. To reduce the molecular weight, hydrogen is added to the polymerization reaction:
- LnTi–CH2−CHR–polymer + H2 → LnTi−H + CH3−CHR–polymer
Another termination process involves the action of protic reagents, which can be intentionally added or adventitious.
Commercial polymers prepared with Ziegler–Natta catalysts
- Polyethylene
- Polypropylene
- Copolymers of ethylene and 1-alkenes
- Polybutene-1
- Polymethylpentene
- Polycycloolefins
- Polybutadiene
- Polyisoprene
- Amorphous poly-alpha-olefins (APAO)
- Polyacetylene
References
- Giuliano Cecchin, Giampiero Morini, Fabrizio Piemontesi (2003). "Ziegler-Natta Catalysts". Ziegler–Natta Catalysts. Kirk-Othmer Encyclopedia of Chemical Technology. Wiley-VCH. doi:10.1002/0471238961.2609050703050303.a01. ISBN 0471238961.CS1 maint: uses authors parameter (link)
- Hoff, Ray; Mathers, Robert T., eds. (2010). Handbook of Transition Metal Polymerization Catalysts (Online ed.). John Wiley & Sons. doi:10.1002/9780470504437. ISBN 9780470504437.
- Natta, G.; Danusso, F., eds. (1967). Stereoregular Polymers and Stereospecific Polymerizations. Pergamon Press.
- Nowlin, T. E.; Mink, R. I.; Kissin, Y. V. (2010). "Supported Magnesium/Titanium-Based Ziegler Catalysts for Production of Polyethylene". In Hoff, Ray; Mathers, Robert T. (eds.). Handbook of Transition Metal Polymerization Catalysts. Handbook of Transition Metal Polymerization Catalysts (Online ed.). John Wiley & Sons. pp. 131–155. doi:10.1002/9780470504437.ch6. ISBN 9780470504437.
- Polypropylene Production via Gas Phase Process, Technology Economics Program. Intratec. 2012. ISBN 978-0-615-66694-5.
- Norio Kashiwa (2004). "The Discovery and Progress of MgCl2-Supported TiCl4 Catalysts". Journal of Polymer Science A. 42 (1): 1–8. Bibcode:2004JPoSA..42....1K. doi:10.1002/pola.10962.
- Hill, A. F. (2002). Organotransition Metal Chemistry. New York: Wiley-InterScience. pp. 136–139.
- Kissin, Y. V. (2008). "Chapter 4". Alkene Polymerization Reactions with Transition Metal Catalysts. Amsterdam: Elsevier.
- Klosin, J.; Fontaine, P. P.; Figueroa, R. (2015). "Development of Group Iv Molecular Catalysts for High Temperature Ethylene-Α-Olefin Copolymerization Reactions". Accounts of Chemical Research. 48 (7): 2004–2016. doi:10.1021/acs.accounts.5b00065. PMID 26151395.
- Bochmann, M. (1994). Organometallics 1, Complexes with Transition Metal-Carbon σ-Bonds. New York: Oxford University Press. pp. 69–71. ISBN 9780198558132.
- Alt, H. G.; Koppl, A. (2000). "Effect of the Nature of Metallocene Complexes of Group IV Metals on Their Performance in Catalytic Ethylene and Propylene Polymerization". Chem. Rev. 100 (4): 1205–1222. doi:10.1021/cr9804700. PMID 11749264.
- Elschenbroich, C.; Salzer, A. (1992). Organometallics: a Concise Introduction. New York: VCH Verlag. pp. 423–425.
Further reading
- Kissin, Y. V. (2008). Alkene Polymerization Reactions with Transition Metal Catalysts. Amsterdam: Elsevier.
- Corradini, P.; Guerra, G.; Cavallo, L. (2004). "Do New Century Catalysts Unravel the Mechanism of Stereocontrol of Old Ziegler–Natta Catalysts?". Acc. Chem. Res. 37 (4): 231–241. doi:10.1021/ar030165n. PMID 15096060.
- Takahashi, T. (2001). "Titanium(IV) Chloride-Triethylaluminum". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons.
- Britovsek, G. J. P.; Gibson, V. C.; Wass, D. F. (1999). "The Search for New-Generation Olefin Polymerization Catalysts: Life beyond Metallocenes". Angew. Chem. Int. Ed. 38 (4): 428–447. doi:10.1002/(SICI)1521-3773(19990215)38:4<428::AID-ANIE428>3.0.CO;2-3. PMID 29711786.