Baloxavir marboxil
Baloxavir marboxil (BXM), sold under the brand name Xofluza, is an antiviral medication for treatment of influenza A and influenza B flu.[3] It was approved for medical use both in Japan and in the United States in 2018,[4][5] and is taken as a single dose by mouth.[3] It may reduce the duration of flu symptoms by about a day, but is prone to selection of resistant mutants that render it ineffectual.[6]
 | |
| Clinical data | |
|---|---|
| Trade names | Xofluza |
| Other names | BXM (S-033188) BXA (S-033447) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618062 |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
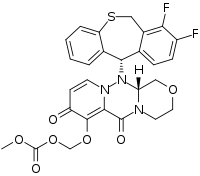
| Formula | C27H23F2N3O7S |
| Molar mass | 571.55 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Baloxavir marboxil was developed as a prodrug strategy, with its metabolism releasing the active agent, baloxavir acid (BXA). BXA then functions as enzyme inhibitor, targeting the influenza virus' cap-dependent endonuclease activity, used in "cap snatching" by the virus' polymerase complex, a process essential to its life-cycle.[7]
BXM was developed for the market by Shionogi Co., a Japanese pharmaceutical company, and Switzerland-based Roche AG.[8] The names under which BXM and BXA appear in Shionogi research reporting are S-033188 and S-033447, respectively.
Medical uses
Baloxavir marboxil is an influenza medication, an antiviral,[9][3] that is taken as a single dose tablet,[9] by mouth, by individuals that are 12 years of age or older,[9] that have presented symptoms of this infection for no more than 48 hours.[9][10] The efficacy of baloxavir marboxil administered after 48 hours has not been tested.[9]
Resistance
In 2.2% of baloxavir recipients in the Phase II trial and in about 10% of baloxavir recipients in the Phase III trial, the infecting influenza strain had acquired resistance to the drug, due to variants of the polymerase protein displaying substitutions of isoleucine-38, specifically, the I38T, I38M, or I38F mutations.[11] There is continuing research into and clinical concern over the resistance appearing in patients, in response to treatment with this drug.[12][13][14]
Side effects
Common side effects following the single dose administration of baloxavir were diarrhea, bronchitis, common cold, headache, and nausea.[3][10][4] Adverse events were reported in 21% of people who received baloxavir, 25% of those receiving placebo, and 25% of oseltamivir.[11]
Mechanism of action
Baloxavir marboxil is an influenza therapeutic agent, specifically, an enzyme inhibitor targeting the influenza virus' cap-dependent endonuclease activity, one of the activities of the virus polymerase complex.[15] In particular, it inhibits a process known as cap snatching, by which the virus derives short, capped primers from host cell RNA transcripts, which it then uses for polymerase-catalyzed synthesis of its needed viral mRNAs.[16] A polymerase subunit binds to the host pre-mRNAs at their 5′-caps, then the polymerase's endonuclease activity catalyzes its cleavage "after 10–13 nucleotides".[16] As such, its mechanism is distinct from neuraminidase inhibitors such as oseltamivir and zanamivir.
Chemistry
Baloxavir marboxil is a substituted pyridone derivative of a polycyclic family, whose chemical synthesis has been reported in a number of ways by the company discovering it, Shionogi and Co. of Japan (as well as others); the Shionogi reports have appeared several times in the Japanese patent literature between 2016 and 2019, providing insight into possible industrial synthetic routes that may be in use.[17][18][19][20][21][22]
Baloxavir marboxil (BXM) is a prodrug whose active agent, baloxavir acid (BXA) is released rapidly in vivo, as the hydrolysis of BXM is catalysed by arylacetamide deacetylases in cells of the blood, liver, and lumen of the small intestine.[23][24][25] The compound numbers for BXM and BXA used in publications by Shionogi and others during discovery and development (prior to assignment of a United States Adopted Name (USAN)) were, respectively, S-033188 and S-033447.[26] As reported in a review of the patent literature, the carbonic acid ester (carbonate) moiety of the prodrug—shown in the lower left-hand corner of the image above—was prepared during discovery and development research from a late stage 2-hydroxy-4-pyridone precursor by treatment with chloromethyl methyl carbonate.[23][27]
Approval
Japan's Ministry of Health, Labour and Welfare (JMHLW) and the U.S. Food and Drug Administration (FDA) approved baloxavir marboxil based on evidence of its benefits and side effects from two clinical trials in adult and pediatric patients with uncomplicated influenza (Trial 1, 1518T0821 and Trial 2, NCT02954354[28]),[3] involving 1119 patients.[10][29] Both trials included clincal sites and patients in Japan, with Trial 2 adding clinical locations in the United States.[3][10]
As of September 2018, in the only report of a Phase III randomized, controlled trial, baloxavir reduced the duration of influenza symptoms of otherwise healthy outpatients by about one day compared with a placebo treatment group, and comparable with what was seen for an oseltamivir treatment group.[10] On the first day after baloxavir was started in its treatment group of patients, viral loads decreased more than in patients in either the oseltamivir or placebo groups; however, after five days, the effect on viral load of the single dose of baloxavir was indistinguishable from the effect observed following the complete, 5-day regimen of oseltamivir in its treatment group.[11]
Baloxavir marboxil was approved for sale in Japan in February 2018.[30] On 24 October 2018, the FDA approved it for the treatment of acute uncomplicated influenza in people 12 years of age and older who have been symptomatic for no more than 48 hours.[4] The FDA application of baloxavir marboxil was granted priority review in the United States, and approval of Xofluza was granted to Shionogi & Co., Ltd. in October 2019.[4][5] Specifically, the FDA approved the use of baloxavir marboxil for people at high risk of developing influenza-related complications.[31]
Baloxavir marboxil was approved for medical use in Australia in February 2020.[1]
Research
While it is being studied in COVID-19, no published evidence supports its use as of 8 April 2020.[32][33]
References
- "Xofluza Australian prescription medicine decision summary". Therapeutic Goods Administration (TGA). 2 March 2020. Retrieved 16 August 2020.
- "Baloxavir marboxil (Xofluza) Use During Pregnancy". Drugs.com. 14 November 2018. Retrieved 9 January 2020.
- "Xofluza- baloxavir marboxil tablet, film coated". DailyMed. 28 October 2019. Retrieved 9 January 2020.
- "FDA approves new drug to treat influenza". U.S. Food and Drug Administration (FDA) (Press release). 24 October 2018. Archived from the original on 24 October 2019. Retrieved 23 October 2019.

- "Drug Approval Package: Xofluza Film-Coated Tablets (Baloxavir marboxil)". U.S. Food and Drug Administration (FDA). 7 December 2018. Retrieved 10 May 2020.
- "Baloxavir Marboxil for Uncomplicated Influenza: Mechanism of Action & Side Effects". Huateng Pharma. Retrieved 29 May 2020.
- Noshi T, Kitano M, Taniguchi K, et al. (December 2018). "In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit". Antiviral Res. 160: 109–117. doi:10.1016/j.antiviral.2018.10.008. PMID 30316915.
- Branswell H (27 June 2018). "A flu drug — shown to reduce the duration of symptoms — could upend treatment in U.S.". Stat.
- "Baloxavir Marboxil Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. 19 August 2019. Retrieved 9 January 2020.
- Food and Drug Administration (23 July 2019). "Drug Trial Snapshot: Xofluza". U.S. Food and Drug Administration (FDA). Retrieved 7 January 2020.

- Hayden FG, Sugaya N, Hirotsu N, et al. (September 2018). "Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents". N. Engl. J. Med. 379 (10): 913–923. doi:10.1056/NEJMoa1716197. PMID 30184455.
- "Roche's flu med Xofluza drives drug resistance and may be a bad choice for kids, study says". FiercePharma.
- "New flu drug drives drug resistance in influenza viruses". EurekAlert!.
- Imai M, Yamashita M, Sakai-Tagawa Y, et al. (January 2020). "Influenza A variants with reduced susceptibility to baloxavir isolated from Japanese patients are fit and transmit through respiratory droplets". Nature Microbiology. 5 (1): 27–33. doi:10.1038/s41564-019-0609-0. PMID 31768027.
- Eisfeld AJ, Neumann G, Kawaoka Y (January 2015). "At the centre: influenza A virus ribonucleoproteins". Nature Reviews. Microbiology. 13 (1): 28–41. doi:10.1038/nrmicro3367. PMC 5619696. PMID 25417656.
- Dias A, Bouvier D, Crépin T, et al. (April 2009). "The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit". Nature. 458 (7240): 914–8. Bibcode:2009Natur.458..914D. doi:10.1038/nature07745. PMID 19194459.
- MedKoo Staff (8 January 2020). "Baloxavir marboxil: Synthetic Routes [Five Tabs]". Hodoodo.com. Morrisville, NC: MedKoo Biosciences. Retrieved 8 January 2020.
- WO 2019070059, Okamoto K, Ueno T, Hato Y, Hakogi T, Majima S, "Method for stereoselective preparation of substituted polycyclic pyridone derivative.", published 2019, assigned to Shionogi & Co., Ltd., Japan.
- JPWO 2018030463, Kawai M, Tomita K, Akiyama T, Okano A, Miyagawa M, "Pharmaceutical composition containing polycyclic pyridone derivatives as cap-dependent endonuclease (CEN) inhibitors.", published 2017, assigned to Shionogi and Co., Ltd., Japan.
- JPWO 2017221869, Shibahara S, Fukui N, Maki T, "Polycyclic pyridone derivative, crystal and preparation method thereof.", published 2017, assigned to Shionogi and Co., Ltd., Japan.
- JPWO 2016175224, Kawai M, Tomita K, Akiyama T, Okano A, Miyagawa M, "Preparation of polycyclic pyridone derivatives as cap-dependent endonuclease (CEN) inhibitors and prodrugs thereof.", published 2016, assigned to Shionogi and Co., Ltd., Japan
- CN 108440564, Zhu X, Jiang W, "Process for preparation of substituted polycyclic carbamoylpyridone derivative and its prodrug.", published 2018
- Hughes DL (21 June 2019). "Review of the Patent Literature: Synthesis and Final Forms of Antiviral Drugs Tecovirimat and Baloxavir Marboxil". Organic Process Research & Development. 23 (7): 1298–1307. doi:10.1021/acs.oprd.9b00144.
- Yoshino R, Yasuo N, Sekijima M (November 2019). "Molecular Dynamics Simulation reveals the mechanism by which the Influenza Cap-dependent Endonuclease acquires resistance against Baloxavir marboxil". Scientific Reports. 9 (1): 17464. Bibcode:2019NatSR...917464Y. doi:10.1038/s41598-019-53945-1. PMID 31767949.
- Kawaguchi N, Koshimichi H, Ishibashi T, Wajima T (November 2018). "Evaluation of Drug-Drug Interaction Potential between Baloxavir Marboxil and Oseltamivir in Healthy Subjects". Clinical Drug Investigation. 38 (11): 1053–1060. doi:10.1007/s40261-018-0697-2. PMID 30203386.
- Yang J, Huang Y, Liu S (May 2019). "Investigational antiviral therapies for the treatment of influenza". Expert Opinion on Investigational Drugs. 28 (5): 481–488. doi:10.1080/13543784.2019.1606210. PMID 31018720.
- "Baloxavir Marboxil". Pharmaceutical Substances.
- Clinical trial number NCT02954354 for "A Study of S-033188 (Baloxavir Marboxil) Compared With Placebo or Oseltamivir in Otherwise Healthy Patients With Influenza (CAPSTONE 1)" at ClinicalTrials.gov
- Otake T (24 February 2018). "One-dose flu drug Xofluza gets nod from health ministry". Japan Times Online.
- "Xofluza (Baloxavir Marboxil) Tablets 10mg/20mg Approved For The Treatment Of Influenza Types A And B In Japan" (Press release). Shionogi & Co., Ltd. 23 February 2018 – via publicnow.com.
- "Genentech Announces FDA Approval of Xofluza (Baloxavir Marboxil) for People at High Risk of Developing Influenza-Related Complications" (Press release). Genentech. 17 October 2019. Archived from the original on 24 October 2019. Retrieved 23 October 2019 – via Business Wire.
- "Assessment of Evidence for COVID-19-Related Treatments: Updated 4/3/2020". ASHP. Retrieved 7 April 2020.
- "Coronavirus COVID-19 (SARS-CoV-2)". Johns Hopkins ABX Guide. Retrieved 12 April 2020.
Further reading
- Ikematsu H, Hayden FG, Kawaguchi K, Kinoshita M, de Jong MD, Lee N, et al. (July 2020). "Baloxavir Marboxil for Prophylaxis against Influenza in Household Contacts". N. Engl. J. Med. 383 (4): 309–320. doi:10.1056/NEJMoa1915341. PMID 32640124.
- Mushtaq A (December 2018). "Baloxavir: game-changer or much ado about nothing?". Lancet Respir Med. 6 (12): 903–904. doi:10.1016/S2213-2600(18)30469-7. PMID 30420246. Short review characterizing strengths and weaknesses of the medication, including the matters of single dose and selected resistance.
- Rana P (10 February 2018). "Experimental Drug Promises to Kill the Flu Virus in a Day". The Wall Street Journal. Retrieved 7 January 2020. An early report in the business news, overstating the claim and over-elevating the expectations for the treatment.
External links
- "Baloxavir marboxil". Drug Information Portal. U.S. National Library of Medicine.
- "Baloxavir marboxil". SPS - Specialist Pharmacy Service.