Thiosemicarbazide
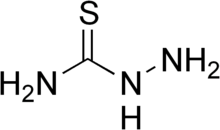
Thiosemicarbazide is the chemical compound with the formula H2NC(S)NHNH2. A white, odorless solid, it is related to thiourea (H2NC(S)NH2) by the insertion of an NH center. They are commonly used as ligands for transition metals.[1] Many thiosemicarbazides are known. These feature an organic substituent in place of one or more H's of the parent molecule. 4-Methyl-3-thiosemicarbazide is a simple example.
 | |
| Names | |
|---|---|
| Other names
hydrazinecarbothioamide, N-aminothiourea, aminothiourea | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.077 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 2811 2771 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH5N3S | |
| Molar mass | 91.13 g·mol−1 |
| Appearance | white solid |
| Density | 1.465 g/cm3 |
| Melting point | 183 °C (361 °F; 456 K) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Danger |
GHS hazard statements |
H300, H412 |
| P264, P270, P273, P301+310, P321, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
According to X-ray crystallography, the CSN3 core of the molecule is planar as are the three H's nearest the thiocarbonyl group.[2]
Reactions
Thiosemicarbazides are precursors to thiosemicarbazones. They are precursors to heterocycles.[3] Formylation of thiosemicarbazide provides access to triazole.[4]
gollark: I just make it turn off my computer's webcam, so that the universe isn't observed and thus no longer exists. phyzic.
gollark: And if you're root you can already do *basically* anything else anyway.
gollark: You need to be root.
gollark: No.
gollark: I think so?
References
- Campbell, Michel J.M. (1975). "Transition metal complexes of thiosemicarbazide and thiosemicarbazones". Coordination Chemistry Reviews. 15 (2–3): 279–319. doi:10.1016/S0010-8545(00)80276-3.
- Andreetti, G. D.; Domiano, P.; Gasparri, G. F.; Nardelli, M.; Sgarabotto, P. (1970). "Hydrogen bonding in thiosemicarbazide". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 26 (7): 1005–1009. doi:10.1107/S0567740870003497.
- Gazieva, Galina A.; Kravchenko, Angelina N. (2012). "Thiosemicarbazides in the synthesis of five- and six-membered heterocyclic compounds". Russian Chemical Reviews. 81 (6): 494–523. Bibcode:2012RuCRv..81..494G. doi:10.1070/RC2012v081n06ABEH004235.
- C. Ainsworth (1960). "1,2,4-Triazole". Organic Syntheses. 40: 99. doi:10.15227/orgsyn.040.0099.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.