Thiosemicarbazone
A thiosemicarbazone is an organosulfur compound with the formula H2NC(S)NHN=CR2. Many variations exist, including those where some or all of the NH centers are substituted by organic groups. Thiosemicarbazones are usually produced by condensation of a thiosemicarbazide with an aldehyde or ketone:
- H2NC(S)NHNH2 + O=CR2 → H2NC(S)NHN=CR2 + H2O
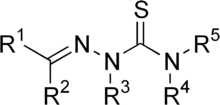
General chemical structure of a thiosemicarbazone
In terms of their chemical structures, the CSN3 core atoms are coplanar.[1]
Occurrence and applications
Some thiosemicarbazones have medicinal properties, e.g. the antiviral metisazone and the antibiotic thioacetazone. Thiosemicarbazones are also widely used as ligands in coordination chemistry.[2] The affinity of thiosemicarbazones for metal ions is exploited in controlling iron overload.[3]
gollark: Observe the advancement of Minoteaur.
gollark: Ah yes, Rust development.
gollark: Annihilation planes can be filtered using filtered storage buses.
gollark: You can connect infinitely many storage devices with one channel by recursively using interface/storage bus things.
gollark: Logistics Pipes would be acceptable.
References
- Wattanakanjana, Yupa; Pakawatchai, Chaveng; Saithong, Saowanit; Piboonphon, Prapaporn; Nimthong, Ruthairat (2012). "Iodido[1-(propan-2-ylidene)thiosemicarbazide-κS]bis(triphenylphosphane-κP)copper(I)". Acta Crystallographica Section E. 68 (11): m1417–m1418. doi:10.1107/S1600536812044066. PMC 3515154. PMID 23284381.
- Campbell, Michel J.M. (1975). "Transition metal complexes of thiosemicarbazide and thiosemicarbazones". Coordination Chemistry Reviews. 15 (2–3): 279–319. doi:10.1016/S0010-8545(00)80276-3.
- Merlot, Angelica M.; Kalinowski, Danuta S.; Richardson, Des R. (2013). "Novel Chelators for Cancer Treatment: Where Are We Now?". Antioxidants & Redox Signaling. 18 (8): 973–1006. doi:10.1089/ars.2012.4540. PMID 22424293.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.