Tetrarhodium dodecacarbonyl
Tetrarhodium dodecacarbonyl is the chemical compound with the formula Rh4(CO)12. This dark-red crystalline solid is the smallest stable binary rhodium carbonyl. It is used as a catalyst in organic synthesis.
12.png) | |
 | |
| Names | |
|---|---|
| IUPAC name
tri-μ-carbonyl-1:2κ2C;1:3κ2C;2:3κ2C-nonacarbonyl-
1κ2C,2κ2C,3κ2C,4κ3C-[Td-(13)-Δ4-closo]- | |
| Other names
rhodium(0) carbonyl; rhodium carbonyl; rhodium dodecacarbonyl | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.039.232 |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| Rh4(CO)12 | |
| Molar mass | 747.743 g/mol |
| Appearance | Red crystals |
| Solubility | Chlorocarbons, toluene, tetrahydrofuran |
| Related compounds | |
Related compounds |
Rhodium(III) chloride, Rh6(CO)16, Rh2(CO)4Cl2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure, synthesis, reactions
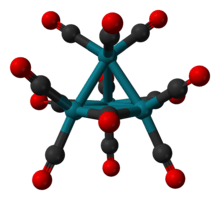
The structure of Rh4(CO)12 is described by a tetrahedral array of four Rh atoms with nine terminal CO ligands and three bridging CO ligands. The structure can be expressed as Rh4(CO)9(µ-CO)3.[1] It is prepared by treatment of an aqueous solution of rhodium trichloride with activated copper metal under an atmosphere of CO.[2]
- 4 RhCl3(H2O)3 + 8 Cu + 22 CO → Rh4(CO)12 + 2 CO2 + 8 Cu(CO)Cl + 4 HCl + 10 H2O
Alternatively, the compound can be prepared by treatment of a methanolic solution of RhCl3(H2O)3 with CO to afford H[RhCl2(CO)2], followed by carbonylation in the presence of sodium citrate.[1]
The cluster undergoes thermal substitution with phosphorus ligands, L:
- Rh4(CO)12-n + n L → Rh4(CO)12-nLn + n CO
Related metal carbonyls
Because of their relevance to hydroformylation catalysis, the metal carbonyls has been systematically studied to a high degree. The instability of Rh2(CO)8 has been a source of curiosity. The analogous binary carbonyl of cobalt, Co2(CO)8, is well known. Solutions of Rh4(CO)12 under high pressures of CO convert to the dirhodium compound:[3]
- Rh4(CO)12 + 4 CO → 2 Rh2(CO)8
Unlike Co2(CO)8 which features bridging carbonyls, the main isomer of Rh2(CO)8 features only terminal CO ligands. The relative instability of Rh2(CO)8 is analogous to the tendency of Ru(CO)5 to convert to Ru3(CO)12.
References
- Serp, P.; Kalck, P.; Feurer, R.; Morancho, R. (1998). Marcetta. Y. Darensbourg (ed.). "Tri-µ-carbonyl-nonacarbonyltetrarhodium". Inorganic Syntheses. Inorganic Syntheses. 32: 284–287. doi:10.1002/9780470132630.ch45. ISBN 9780470132630.
- S. Martinengo; G. Giordano; P. Chini; G. W. Parshall; E. R Wonchoba (1990). Robert J. Angelico (ed.). "Tri-µ-carbonyl-nonacarbonyltetrarhodium". Inorganic Syntheses. Inorganic Syntheses. 28: 242–245. doi:10.1002/9780470132593.ch62. ISBN 9780470132593.
- Brown, D. T.; Eguchi, T.; Heaton, B. T.; Iggo, J. A.; Whyman, R. (1991). "High-pressure spectroscopic studies of reactions of the clusters [Rh4(CO)12–x{P(OPh)3}x] (x = 1–4) with carbon monoxide or syngas". Journal of the Chemical Society, Dalton Transactions: 677–683. doi:10.1039/DT9910000677.
General reading
- King, R. B., "Rhodium: Organometallic Chemistry" Encyclopedia of Inorganic Chemistry 1994, 7, 3494.