Cyclooctadiene rhodium chloride dimer
Cyclooctadiene rhodium chloride dimer is the organorhodium compound with the formula Rh2Cl2(C8H12)2, commonly abbreviated [RhCl(COD)]2 or Rh2Cl2(COD)2. This yellow-orange, air-stable compound is a widely used precursor to homogeneous catalysts.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
di-μ-chlorido- | |
| Other names
Cyclooctadiene rhodium chloride dimer | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.949 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H24Cl2Rh2 | |
| Molar mass | 493.0806 g/mol |
| Density | 1.93 g/cm3 |
| Melting point | 243 °C (469 °F; 516 K) |
| Solubility in other solvents | dichloromethane |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
GHS hazard statements |
H302, H315, H317, H319, H335, H411 |
| P261, P264, P270, P271, P272, P273, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P333+313, P337+313, P362, P363, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and reactions
The synthesis of [RhCl(COD)]2 involves heating a solution of hydrated rhodium trichloride with 1,5-cyclooctadiene in aqueous ethanol in the presence of sodium carbonate:[1][2]
- 2 RhCl3·3H2O + 2 COD + 2 CH3CH2OH + 2 Na2CO3 → [RhCl(COD)]2 + 2 CH3CHO + 8 H2O + 2 CO2 + 4 NaCl
[RhCl(COD)]2 is principally used as a source of the electrophile "[Rh(COD)]+."
- [RhCl(COD)]2 + nL → [LnRh(COD)]+Cl− (where L = PR3, alkene, etc. and n = 2 or 3)
In this way, chiral phosphines such as chiraphos, DIPAMP, and DIOP have been attached to Rh. The resulting chiral catalysts are capable of asymmetrically hydrogenating certain prochiral alkenes.[3] A closely related but still more reactive complex is chlorobis(cyclooctene)rhodium dimer.
The dimer reacts with a variety of Lewis bases (B:) to form adducts B:RhCl(COD).
Its reaction with 1-methylimidazole and the corresponding enthalpy are:
- 1-CH3NC3N: + 1/2Rh2Cl2(COD)2 → 1-CH3NC3N:RhCl(COD) ΔH= -40.kJ mol-1
This enthalpy corresponds to the enthalpy change for a reaction forming one mole of the product, 1-CH3NC3N:RhCl(COD), from the acid dimer. The dissociation energy for cyclooctadiene rhodium chloride dimer, which is an energy contribution prior to reaction with the donor,
- Rh2Cl2(COD)2 →2 RhCl(COD)
has been estimated to be 48 kJ mol-1 by the ECW model.
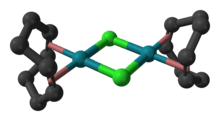
Structure
The molecule consists of a pair of square planar Rh centers bound to a 1,5-cyclooctadiene and two chloride ligands that are shared between the Rh centers. The Rh2Cl2 core is also approximately planar,[4] in contrast to the highly bent structure of cyclooctadiene iridium chloride dimer where the dihedral angle is 86°.
References
- Giordano, G.; Crabtree, R. H. “Di-μ-chloro-bis(η4-1,5-cyclooctadiene)dirhodium(I)” Inorganic Syntheses, 1990, volume 28, pages 88-90. doi:10.1002/9780470132593.ch22
- Chatt, J.; Venanzi, L. M. (1956). "Olefin Complexes of Rhodium". Nature. 177: 852–3. doi:10.1038/177852b0.CS1 maint: uses authors parameter (link)
- W. S. Knowles (2003). "Asymmetric Hydrogenations (Nobel Lecture 2001)". Advanced Synthesis & Catalysis. 345 (1–2): 3. doi:10.1002/adsc.200390028.
- "Di-μ-chloro-bis[(cis,cis-η4-1,5-cyclooctadiene)rhodium(I)]: a redetermination" De Ridder, Kirk J. A. Acta Crystallographica, Section C: Crystal Structure Communications 1994, C50, 1569-72. doi:10.1107/S0108270194001459
External links
