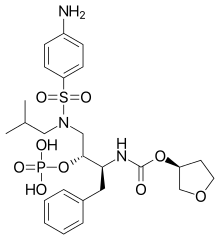
Fosamprenavir
Fosamprenavir (marketed by ViiV Healthcare as the calcium salt under the trade names Lexiva in the U.S. and Telzir in Europe) is a drug for the treatment of HIV infections. It is a pro-drug of the protease inhibitor and antiretroviral drug amprenavir. The FDA approved it October 20, 2003, while the EMA approved it on July 12, 2004. The human body metabolizes fosamprenavir in order to form amprenavir, which is the active ingredient. That metabolization increases the duration that amprenavir is available, making fosamprenavir a slow-release version of amprenavir and thus reducing the number of pills required versus standard amprenavir.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lexiva, Telzir |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604012 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | 90% |
| Metabolism | Hydrolysed to amprenavir and phosphate in GI tract epithelium |
| Elimination half-life | 7.7 hours |
| Excretion | Fecal (as metabolites of amprenavir) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H36N3O9PS |
| Molar mass | 585.608 g/mol 623.700 g/mol (calcium salt) g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
A head-to-head study with lopinavir[1] showed the two drugs to have comparable potency, but patients on fosamprenavir tended to have a higher serum cholesterol. Fosamprenavir's main advantage over lopinavir is that it is cheaper.
Medical uses
Fosamprenavir is used for the treatment of HIV-1 infections, typically but not necessarily in combination with low-dose ritonavir or other antiviral drugs.[2][3]
Adverse effects
The most common adverse effect is diarrhea. Other common side effects include headache, dizziness and exanthema, which is usually transient. Severe allergic reactions (Stevens–Johnson syndrome) are rare.[2]
Interactions
Amprenavir (the active metabolite of fosamrenavir, which is found in blood plasma, liver and other organs) is metabolized via the liver enzyme CYP3A4 and also weakly inhibits this enzyme. This means that combination with drugs that are also metabolized by CYP3A4 can increase their plasma concentrations and thus side effects; and combination with drugs that inhibit CYP3A4 can increase amprenavir concentrations.[2]
When combining fosamprenavir with low doses of the CYP3A4 inhibitor ritonavir, this interaction is intended as it allows for application of lower fosamprenavir doses.[2]
Pharmacology
Fosamprenavir is quickly activated to amprenavir, even before it reaches the circulation. Amprenavir is a HIV protease inhibitor.[2]
References
- Eron J, Yeni P, Gathe J, Estrada V, DeJesus E, Staszewski S, et al. (August 2006). "The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: a randomised non-inferiority trial". Lancet. 368 (9534): 476–82. doi:10.1016/S0140-6736(06)69155-1. PMID 16890834.
- Jasek W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 8009–17. ISBN 978-3-85200-181-4.
- Lexiva. Drugs.com (Report). Monograph.
- Shen CH, Wang YF, Kovalevsky AY, Harrison RW, Weber IT (September 2010). "Amprenavir complexes with HIV-1 protease and its drug-resistant mutants altering hydrophobic clusters". The FEBS Journal. 277 (18): 3699–714. doi:10.1111/j.1742-4658.2010.07771.x. PMC 2975871. PMID 20695887.