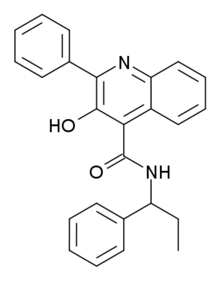
Talnetant
Talnetant (SB-223,412) is a neurokinin 3 receptor antagonist developed by GlaxoSmithKline, which is being researched for several functions (primarily treatment of irritable bowel syndrome, despite a 2007 study finding no statistically significant improvement in rectal hypersensitivity over placebo). Its use as a potential antipsychotic drug for the treatment of schizophrenia has also been discontinued.[1][2][3][4]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.236.526 |
| Chemical and physical data | |
| Formula | C25H22N2O2 |
| Molar mass | 382.463 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
See also
References
- "UPDATE 1-GlaxoSmithKline prunes new drug pipeline". Reuters. Oct 15, 2007.
- Evangelista S (July 2005). "Talnetant GlaxoSmithKline". Current Opinion in Investigational Drugs. 6 (7): 717–21. PMID 16044668.
- Houghton LA, Cremonini F, Camilleri M, Busciglio I, Fell C, Cox V, et al. (September 2007). "Effect of the NK(3) receptor antagonist, talnetant, on rectal sensory function and compliance in healthy humans". Neurogastroenterology and Motility. 19 (9): 732–43. doi:10.1111/j.1365-2982.2007.00934.x. PMID 17727393.
- Dawson LA, Cato KJ, Scott C, Watson JM, Wood MD, Foxton R, et al. (June 2008). "In vitro and in vivo characterization of the non-peptide NK3 receptor antagonist SB-223412 (talnetant): potential therapeutic utility in the treatment of schizophrenia". Neuropsychopharmacology. 33 (7): 1642–52. doi:10.1038/sj.npp.1301549. PMID 17728699.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.