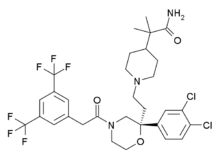
Burapitant
Burapitant (SSR-240,600) is a drug developed by Sanofi-Aventis which was one of the first compounds developed that acts as a potent and selective antagonist for the NK1 receptor.[1][2][3] While burapitant itself did not proceed beyond early clinical trials and was never developed for clinical use in humans, promising animal results from this and related compounds have led to a number of novel drugs from this class that have now been introduced into medical use.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C31H35Cl2F6N3O3 |
| Molar mass | 682.53 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Emonds-Alt X, Proietto V, Steinberg R, Oury-Donat F, Vigé X, Vilain P, et al. (December 2002). "SSR240600 [(R)-2-(1-[2-[4-[2-[3,5-bis(trifluoromethyl)phenyl]acetyl]-2-(3,4-dichlorophenyl)-2-morpholinyl]ethyl]- 4-piperidinyl)-2-methylpropanamide], a centrally active nonpeptide antagonist of the tachykinin neurokinin-1 receptor: I. biochemical and pharmacological characterization". The Journal of Pharmacology and Experimental Therapeutics. 303 (3): 1171–9. doi:10.1124/jpet.102.040162. PMID 12438541.
- Steinberg R, Alonso R, Rouquier L, Desvignes C, Michaud JC, Cudennec A, et al. (December 2002). "SSR240600 [(R)-2-(1-[2-[4-[2-[3,5-bis(trifluoromethyl)phenyl]acetyl]-2-(3,4-dichlorophenyl)-2-morpholinyl]ethyl]-4-piperidinyl)-2-methylpropanamide], a centrally active nonpeptide antagonist of the tachykinin neurokinin 1 receptor: II. Neurochemical and behavioral characterization". The Journal of Pharmacology and Experimental Therapeutics. 303 (3): 1180–8. doi:10.1124/jpet.102.040279. PMID 12438542.
- Pérez S, Tierney A, Deniau JM, Kemel ML (December 2007). "Tachykinin regulation of cholinergic transmission in the limbic/prefrontal territory of the rat dorsal striatum: implication of new neurokinine 1-sensitive receptor binding site and interaction with enkephalin/mu opioid receptor transmission" (PDF). Journal of Neurochemistry. 103 (6): 2153–63. doi:10.1111/j.1471-4159.2007.04944.x. PMID 17949415.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.