Squaric acid
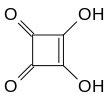
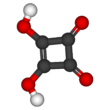
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a dibasic organic acid with the chemical formula C4H2O4.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
3,4-Dihydroxycyclobut-3-ene-1,2-dione | |||
| Other names
Quadratic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.018.875 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H2O4 | |||
| Molar mass | 114.056 g·mol−1 | ||
| Appearance | Gray powder | ||
| Melting point | > 300 °C (572 °F; 573 K) | ||
| Acidity (pKa) | 1.5, 3.4 | ||
| Hazards | |||
| R-phrases (outdated) | R36/37/38 R43 | ||
| S-phrases (outdated) | S26 S36 | ||
| Flash point | 190 °C (374 °F; 463 K)[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
The conjugate base of squaric acid is the hydrogensquarate anion C
4HO−
4; and the conjugate base of the hydrogensquarate anion is the divalent squarate anion C
4O2−
4. This is one of the oxocarbon anions, which consist only of carbon and oxygen.
Squaric acid is a reagent for chemical synthesis, used for instance to make photosensitive squaraine dyes and inhibitors of protein tyrosine phosphatases.
Chemical properties
Squaric acid is a white crystalline powder with a thermal decomposition point of 245 °C at ambient pressure.[3] The onset of thermal decomposition depends on the different thermodynamic conditions such as heating rates.
The structure of squaric acid is not a perfect square, as the carbon–carbon bond lengths are not quite equal. The high acidity with pKa = 1.5 for the first proton and pKa = 3.4 for the second is attributable to resonance stabilization of the anion.[4] Because the negative charges are equally distributed between each oxygen atom, the dianion of squaric acid is completely symmetrical (unlike squaric acid itself) with all C−C bond lengths identical and all C−O bond lengths identical.
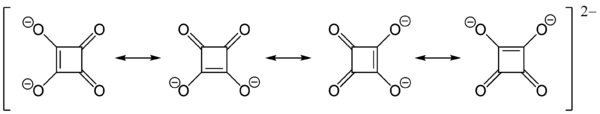
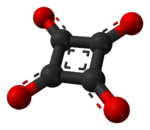
Another, quantum mechanical, way of describing the dianion is to assume that the π electrons of the two C=O double bonds are shifted to the oxygen atoms, so that all four oxygens become single-bonded −O− groups and a double positive electric charge is left in the ring of carbon atoms. In this way the ring fits Hückel's rule for aromaticity (2 π electrons = 4n + 2 with n = 0). The total symmetry of the dianion is a consequence of charge distribution and aromaticity.
Derivatives
Photolysis of squaric acid in a solid argon matrix at 10 K (−263 °C) affords acetylenediol.[5]
Cobalt(II) squarate hydrate Co(C4O4)(H2O)2 (yellow, cubic) can be prepared by autoclaving cobalt(II) hydroxide and squaric acid in water at 200 °C. The water is bound to the cobalt atom, and the crystal structure consists of a cubic arrangement of hollow cells, whose walls are either six squarate anions (leaving a 7 Å wide void) or several water molecules (leaving a 5 Å void).[6]
Cobalt(II) squarate dihydroxide Co3(OH)2(C4O4)2·3H2O (brown) is obtained together with the previous compound. It has a columnar structure including channels filled with water molecules; these can be removed and replaced without destroying the crystal structure. The chains are ferromagnetic; they are coupled antiferromagnetically in the hydrated form, ferromagnetically in the anhydrous form.[6]
Copper(II) squarate monomeric and dimeric mixed-ligand complexes were synthesized and characterized [7]. Infrared, electronic and Q-Band EPR spectra as well as magnetic susceptibilities are reported.
The same method yields iron(II) squarate dihydroxide Fe2(OH)2(C4O4) (light brown).[6]
One or both of the oxygen (=O) groups in the squarate anion can be replaced by other chalcogenides such as sulfur or other divalent groups, such as dicyanomethylene =C(CN)2. The resulting anions, such as 1,2-bis(dicyanomethylene)squarate and 1,3-bis(dicyanomethylene)squarate, retain the aromatic character of squarate and have been called pseudo-oxocarbon anions. There have been theoretical investigations of the analogous compound obtained by substituting amino groups (−NH2) for the hydroxyl (−OH) groups to yield 1,2-diamino-3-cyclobutenedione, and of a compound consisting of two squarate rings bridged by (−NH−) bonds to form bis(3-cyclobutene-1,2-dione)piperazine.[8]
Squaramides are amides of squaric acids. They are four membered vinylogously conjugated diamides. Hydrogen bonding occurs at four locations and is aided by delocalisation of electrons on nitrogen's orbitals to the carbonyl group. Hydrogen bonding and thus delocalization further increases ring aromaticity.[9]
Syntheses
The original synthesis started from reaction of chlorotrifluoroethylene with zinc to perfluorocyclobutene. This compound was converted to 1,2-diethoxy-3,3,4,4-tetrafluoro-1-cyclobutene with ethanol. Hydrolysis gives the squaric acid.[10]
Squarate and related anions such deltate C
3O2−
3 and acetylenediolate C
2O2−
2 have been obtained from carbon monoxide under mild conditions by reductive coupling of CO ligands in organouranium complexes.[11] A similar route recently afforded carbonate anions (in the form of uranium(IV) carbonate) from carbon dioxide.[12]
Medical uses
Medically, SADBE or squaric acid dibutyl ester or dibutyl squarate derives from a squaric acid.[13] is used for the treatment of warts.[14] Squaric acid dibutyl ester is also used for treating alopecia areata or alopecia totalis (autoimmune hair loss) through topical immunotherapy involving the production of an allergic rash.[15] Squaric acid dibutylester is currently undergoing trials for use in treating herpes labialis (cold sores).[16]
Diethylsquarate was utilized in the synthesis of Perzinfotel.
See also
- Cyclobutene, C
4H
6 - Deltic acid, C
3H
2O
3 - Croconic acid, C
5H
2O
5 - Rhodizonic acid, C
6H
2O
6 - Squaramides, the amides of squaric acids
References
- 3,4-Dihydroxy-3-cyclobutene-1,2-dione. Sigma-Aldrich
- 3,4-Dihydroxy-3-cyclobutene-1,2-dione, 98+%. Alfa Aesar
- Lee, K.-S.; Kweon, J. J.; Oh, I.-H.; Lee, C. E. (2012). "Polymorphic phase transition and thermal stability in squaric acid (H
2C
4O
4)". J. Phys. Chem. Solids. 73 (7): 890–895. doi:10.1016/j.jpcs.2012.02.013. - West, Robert; Powell, David L. (1963). "New Aromatic Anions. III. Molecular Orbital Calculations on Oxygenated Anions". J. Am. Chem. Soc. 85 (17): 2577–2579. doi:10.1021/ja00900a010.
- Maier, Günther; Rohr, Christine (1995). "Ethynediol: Photochemical generation and matrix-spectroscopic identification". Liebigs Annalen. 1996 (3): 307–309. doi:10.1002/jlac.15719960304.
- Hitoshi, Kumagai; Hideo, Sobukawa; Mohamedally, Kurmoo (2008). "Hydrothermal syntheses, structures and magnetic properties of coordination frameworks of divalent transition metals". Journal of Materials Science. 43: 2123–2130. doi:10.1007/s10853-007-2033-8.
- J. T. Reinprecht, J. G. Miller, G. C. Vogel, M. S. Haddad and D. N. Hendrickson, (1979), Synthesis and Characterization of Copper(II) Squarate Complexes, Inorg. Chem., 19, 927-931
- Xue, Zhao-Ming; Cheng, Jian-Jun; Chen, Chun-Hua (2006). "Theoretical study of the gas-phase acidity and aromaticity of a novel derivative of nitrogen squaric acid". Journal of Molecular Structure: THEOCHEM. 763 (1–3): 181–186. doi:10.1016/j.theochem.2006.01.026.
- "Squaramides: physical properties, synthesis and applications". Chem. Soc. Rev. 40: 2330–2346. 2011. doi:10.1039/c0cs00200c.
- Park, J. D.; Cohen, S. & Lacher, J. R. (1962). "Hydrolysis Reactions of Halogenated Cyclobutene Ethers: Synthesis of Diketocyclobutenediol". J. Am. Chem. Soc. 84 (15): 2919–2922. doi:10.1021/ja00874a015.
- Frey, Alistair S.; Cloke, F. Geoffrey N.; Hitchcock, Peter B. (2008). "Mechanistic Studies on the Reductive Cyclooligomerisation of CO by U(III) Mixed Sandwich Complexes; the Molecular Structure of [(U(η-C8H6{Si′Pr3-1,4}2)(η-Cp*)]2(μ-η1:η1-C2O2)". Journal of the American Chemical Society. 130 (42): 13816–13817. doi:10.1021/ja8059792.
- Summerscales, Owen T.; Frey, Alistair S. P.; Cloke, F. Geoffrey N.; Hitchcock, Peter B. (2009). "Reductive disproportionation of carbon dioxide to carbonate and squarate products using a mixed-sandwich U(III) complex". Chemical Communications (2): 198–200. doi:10.1039/b815576c.
- "Squaric acid dibutyl ester".
- "Warts". Wilmington Dermatology Center. Retrieved 2011-10-23.
- Holzer, A. M.; Kaplan, L. L.; Levis, W. R. (2006). "Haptens as drugs: contact allergens are powerful topical immunomodulators". J. Drugs Dermatol. 5 (5): 410–416. PMID 16703776.
- http://clinicaltrials.gov/show/NCT01971385