Deltic acid
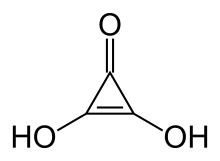
Deltic acid or dihydroxycyclopropenone is a chemical substance with the chemical formula C3O(OH)2. It can be viewed as a ketone and double alcohol of cyclopropene. At room temperature, it is a stable white solid, soluble in diethyl ether, that decomposes (sometimes explosively) between 140 °C and 180 °C, and reacts slowly with water.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,3-dihydroxycycloprop-2-en-1-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H2O3 | |
| Molar mass | 86.05 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The synthesis of deltic acid was first described in 1975 by David Eggerding and Robert West.[2]
Derivatives
Deltate and salts
Deltic acid is considered an acid because it is a particularly acidic enediol, with hydroxyl groups relatively easily losing their protons (pKa1 = 2.57, pKa2 = 6.03), leaving behind the symmetric deltate anion, C
3O2−
3.
The first deltate salts (of lithium and potassium) were described in 1976, also by Eggerding and West. Lithium deltate Li2C3O3 is a water-soluble white solid.[1] Like the other cyclic dianions with formula (CO)2−
n, the deltate anion has a pronounced aromatic character which contributes to its relative stability.[1]
Analogs
An analog of the deltate anion can be obtained by replacing the three oxygen atoms (=O or −O−) by cyanoimino groups (=N−C≡N or −N=C=N−) to yield the symmetric anion C
3(NCN)2−
3.[3] Replacement of the three oxygen atoms by dicyanomethylene (=C(CN)2) provides an oxidizing species that is readily reduced to a stable radical anion and dianion.[4]
Synthesis
Deltic acid was originally obtained by photolysis of the ester bis(trimethylsilyl) squarate, which by loss of one carbonyl group (CO) from the ring was turned into bis(trimethylsilyl) deltate. Decomposition of the latter by butanol yielded deltic acid.[2]
The acid can also be prepared by reaction of silver squarate and trimethylsilyl chloride.[1][5]
Recently the deltate anion has been obtained by direct cyclotrimerization of carbon monoxide at ambient conditions. Carbon monoxide dissolved in pentane reacted with a uranium coordination compound yielding a deltate anion bound to two uranium atoms.[6]
References
- Eggerding, David; West, Robert (1976). "Synthesis and properties of deltic acid (dihydroxycyclopropenone) and the deltate ion". Journal of the American Chemical Society. 98: 3641–3644. doi:10.1021/ja00428a043.
- Eggerding, David; West, Robert (1975). "Synthesis of dihydroxycyclopropenone (deltic acid)". Journal of the American Chemical Society. 97 (1): 207–208. doi:10.1021/ja00834a047.
- Beck, Johannes; Krieger-Beck, Petra (2006). "Crystal structure of 1,2-bis(cyanoimino)-3-triethylammonio-cyclopropenylide". Analytical Sciences. 22: x239. doi:10.2116/analscix.22.x239.
- Fukunaga, T. (1976). "Negatively substituted trimethylenecyclopropane dianions". Journal of the American Chemical Society. 98 (2): 610–611. doi:10.1021/ja00418a050.
- Reetz, M. T.; Neumeier, G.; Kaschube, M. (1975). "Thermische Umlagerung von Quadratsäure-Bis(trimethylsilyl)ester" [Thermal rearrangement of squaric acid bis(trimethylsilyl) ester]. Tetrahedron Letters: 1295. doi:10.1016/S0040-4039(00)72653-0.
- Summerscales, O. T.; Cloke, F. G. N.; Hitchcock, P. B.; N. Hazari, J. C. Green (2006). "Reductive cyclotrimerization of carbon monoxide to the deltate dianion by an organometallic uranium complex". Science. 311 (5762): 829–831. doi:10.1126/science.1121784.