Pneumococcal conjugate vaccine
Pneumococcal conjugate vaccine (PCV) is a pneumococcal vaccine and a conjugate vaccine used to protect infants, young children, and adults against disease caused by the bacterium Streptococcus pneumoniae (pneumococcus). It contains purified capsular polysaccharide of pneumococcal serotypes conjugated to a carrier protein to improve antibody response compared to the pneumococcal polysaccharide vaccine. The World Health Organization (WHO) recommends the use of the conjugate vaccine in routine immunizations given to children.[1]
| Vaccine description | |
|---|---|
| Target disease | Streptococcus pneumoniae |
| Type | Conjugate vaccine |
| Clinical data | |
| Trade names | Prevnar 13, Synflorix, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607021 |
| Routes of administration | Intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| ChemSpider |
|
| KEGG | |
| | |
The most common side effects in children are decreased appetite, fever (only very common in children aged six weeks to five years), irritability, reactions at the site of injection (reddening or hardening of the skin, swelling, pain or tenderness), somnolence (sleepiness) and poor quality sleep.[2] In adults and the elderly, the most common side effects are decreased appetite, headaches, diarrhea, fever (only very common in adults aged 18 to 29 years), vomiting (only very common in adults aged 18 to 49 years), rash, reactions at the site of injection, limitation of arm movement, arthralgia and myalgia (joint and muscle pain), chills and fatigue.[2]
There are two types of PCV available with the brand names Prevnar 13 and Synflorix.[2][3] The brand Pneumosil was prequalified by the WHO in 2020.
Brands
Pneumosil
Pneumosil is a decavalent pneumococcal conjugate vaccine produced by the Serum Institute of India. It contains the serotypes 1, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, and 23F, and was prequalified by WHO in January 2020.[4][5]
Prevnar

Prevnar 13 (PCV13) is produced by Wyeth and replaced Prevnar. It is a tridecavalent vaccine, it contains thirteen serotypes of pneumococcus (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) which are conjugated to diphtheria carrier protein.[6] Prevnar 13 was approved for use in the European Union in December 2009.[2] On February 2010, Prevnar 13 was approved in the United States to replace the pneumococcal 7-valent conjugate vaccine.[7][8] After waiting for the outcome of a trial underway in the Netherlands, the Centers for Disease Control and Prevention (CDC) recommended the vaccine for adults over age 65 in August 2014.[9]
Prevnar (PCV7) was a heptavalent vaccine, meaning that it contains the cell capsule sugars of seven serotypes of the bacteria S. pneumoniae (4, 6B, 9V, 14, 18C, 19F, and 23F), conjugated with diphtheria proteins. It was manufactured by Wyeth (which was acquired by Pfizer).[10] Prevnar was approved for use in the United States in February 2000,[11] and vaccination with Prevnar was recommended for all children younger than two years, and for unvaccinated children between 24 and 59 months old who were at high risk for pneumococcal infections.[12]
Prevnar was produced from the seven most prevalent strains of Streptococcus pneumoniae bacteria in the U.S. The bacterial capsule sugars, a characteristic of these pathogens, are linked to CRM197, a nontoxic recombinant variant of diphtheria toxin (Corynebacterium diphtheriae).
The vaccine's polysaccharide sugars are grown separately in soy peptone broths. Through reductive amination, the sugars are directly conjugated to the protein carrier CRM197 to form the glycoconjugate. CRM197 is grown in C. diphtheriae strain C7 in a medium of casamino acids and yeast extracts.[13]
The original seven-valent formulation contained serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, and resulted in a 98% probability of protection against these strains, which caused 80% of the pneumococcal disease in infants in the U.S. PCV7 is no longer produced.[14]
In 2010, Pfizer introduced Prevnar 13, which contains six additional strains (1, 3, 5, 6A, 19A and 7F), which protect against the majority of the remaining pneumococcal infections.[15]
In March 2020, Pfizer announced its intent to file an adult indication for its 20-valent pneumococcal conjugate vaccine candidate 20vPnC with the Food and Drug Administration following results from a Phase III clinical trial.[16] This candidate contains the serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F.
Synflorix
Synflorix (PCV10) is produced by GlaxoSmithKline. It is a decavalent vaccine, it contains ten serotypes of pneumococcus (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F) which are conjugated to a carrier protein. Synflorix received a positive opinion from the European Medicines Agency for use in the European Union in January 2009[17] and GSK received European Commission authorization to market Synflorix in March 2009.[18][3]
A pentadecavalent vaccine candidate, PCV15 with serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19F, 19A, 22F, 23F, and 33F, has been developed by GlaxoSmithKline and was moved to Phase III clinical trial in 2018.[19]
Schedule of vaccination
As with all immunizations, whether it is available or required, and under what circumstances, varies according to the decisions made by local public health agencies.
Children under the age of two years fail to mount an adequate response to the 23-valent adult vaccine, and so a pneumococcal conjugate vaccine is used. While this covers only seven strains out of more than ninety strains, these seven strains cause 80% to 90% of cases of severe pneumococcal disease, and it is considered to be nearly 100% effective against these strains.[20]
United Kingdom
The UK childhood vaccination schedule consists of a primary course of two doses at 2 and 4 months of age with a final third dose aged 13 months.[21]
Children at special risk (e.g., sickle cell disease and asplenia) require as full protection as can be achieved using the conjugated vaccine, with the more extensive polysaccharide vaccine given after the second year of life:
| Age | 2–6 months | 7–11 months | 12–23 months |
| Conjugated vaccine | 3 × monthly dose | 2 × monthly dose | 2 doses, 2 months apart |
| Further dose in second year of life | |||
| 23-valent vaccine | Then after 2nd birthday single dose of 23-valent | ||
United States
In 2001, the Centers for Disease Control and Prevention (CDC), upon advice from its Advisory Committee on Immunization Practices, recommended the vaccine be administered to every infant and young child in the United States. The resulting demand outstripped production, creating shortages not resolved until 2004. All children, according to the U.S. vaccination schedule, should receive four doses, at two months, four months, six months, and again between one year and fifteen months of age.[22]
Efficacy
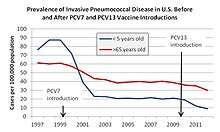
Prevnar-7 is designed to stop seven of about ninety pneumococcal serotypes which have the potential to cause invasive pneumococcal disease (IPD). In 2010, a 13-valent vaccine was introduced. Each year, IPD kills approximately one million children worldwide.[24] Since approval, Prevnar's efficacy in preventing IPD has been documented by a number of epidemiologic studies.[25][26][27] There is evidence that other people in the same household as a vaccinee also become relatively protected.[28] There is evidence that routine childhood vaccination reduces the burden of pneumococcal disease in adults and especially high-risk adults, such as those living with HIV/AIDS.[29]
The vaccine is, however, primarily developed for the U.S. and European epidemiological situation, and therefore it has only a limited coverage of serotypes causing serious pneumococcal infections in most developing countries.[30]
Evidence supporting addition to routine vaccination schedules
After introduction of the pneumococcal conjugate vaccine in 2000, several studies described a decrease in invasive pneumococcal disease in the United States. One year after its introduction, a group of investigators found a 69% drop in the rate of invasive disease in those of less than two years of age.[25] By 2004, all-cause pneumonia admission rates had declined by 39% (95% CI 22–52) and rates of hospitalizations for pneumococcal meningitis decreased by 66% (95% CI 56.3-73.5) in children younger than 2.[31][32]
Rates of invasive pneumococcal disease among adults have also declined since the introduction of the vaccine.[25][32]
Vaccination in low-income countries
Pneumococcal disease is the leading vaccine-preventable killer of young children worldwide, according to the World Health Organization (WHO). It killed more than 500,000 children younger than five years of age in 2008 alone.[33] Approximately ninety percent of these deaths occur in the developing world.[33] Historically 15–20 years pass before a new vaccine reaches one quarter of the population of the developing world.[34]
Pneumococcal vaccines Accelerated Development and Introduction Plan (PneumoADIP) was a GAVI Alliance (GAVI) funded project to accelerate the introduction of pneumococcal vaccinations into low-income countries through partnerships between countries, donors, academia, international organizations and industry. GAVI continues this work and as of March 2013, 25 GAVI-eligible and supported countries have introduced the pneumococcal conjugate vaccine. Further, 15 additional GAVI countries have plans to introduce the vaccine into their national immunization program and 23 additional countries have approved GAVI support to introduce the vaccine.[35]
Sales
Prevnar was among Wyeth's top revenue producers, with sales in 2005 of $1.5 billion, up 43 percent from 2004.[36]
References
- World Health Organization (2019). "Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper –February 2019". Wkly Epidemiol Rec. 94 (8): 85–104. hdl:10665/310970. Lay summary (PDF).
- "Prevenar 13 EPAR". European Medicines Agency (EMA). 26 March 2020. Retrieved 26 March 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Synflorix EPAR". European Medicines Agency. Retrieved 13 July 2020.
- "Gavi-supported pneumococcal conjugate vaccines profiles to support country decision making" (PDF). GAVI. 2019. Retrieved 8 April 2020.
- "Pneumosil, the new pneumococcal vaccine, achieves WHO prequalification, a key step toward improving access and affordability" (Press release). Serum Institute of India. PR Newswire. 28 January 2020. Retrieved 8 April 2020.
- "Prevnar 13". U.S. Food and Drug Administration (FDA). 1 March 2018. STN 125324. Archived from the original on 27 November 2019. Retrieved 27 November 2019.

- "FDA Approves Pneumococcal Disease Vaccine with Broader Protection" (Press release). Archived from the original on 11 September 2010. Retrieved 9 September 2010.

- "Prevnar 13". U.S. Food and Drug Administration (FDA). 12 March 2010. Archived from the original on 12 March 2010. Retrieved 27 November 2019.CS1 maint: unfit url (link)

- "Advisory Committee on Immunization Practices Votes to Recommend Pfizer's Prevnar 13 Vaccine in Adults Aged 65 Years and Older". MarketWatch.com. 13 August 2014. Retrieved 27 April 2017.
- "Pneumococcal 7-valent Conjugate Vaccine (Diphtheria CRM197 Protein)". Wyeth. 2006. Archived from the original on 15 June 2006.
- "February 17, 2000 Approval Letter". U.S. Food and Drug Administration (FDA). Archived from the original on 10 July 2009.

- "American Academy of Pediatrics. Committee on Infectious Diseases. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis". Pediatrics. 106 (2 Pt 1): 362–6. 2000. doi:10.1542/peds.106.2.362. PMID 10920169.
- "Archived copy". Archived from the original on 11 December 2007. Retrieved 21 November 2007.CS1 maint: archived copy as title (link)
- WHO SAGE evidence to recommendations table
- Centers for Disease Control and Prevention (CDC) (March 2010). "Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children — Advisory Committee on Immunization Practices (ACIP), 2010". MMWR Morb. Mortal. Wkly. Rep. 59 (9): 258–61. PMID 20224542.
- "Pfizer Announces Top-Line Results from Phase 3 Study of 20-Valent Pneumococcal Conjugate Vaccine in Pneumococcal Vaccine-Naïve Adults Aged 18 Years or Older" (Press release). Pfizer Inc. 18 March 2020. Retrieved 6 April 2020.
- "EMEA Document" (PDF). Emea.europa.eu. Archived from the original (PDF) on 19 February 2009. Retrieved 27 April 2017.
- "GSK Release". Gsk.com. Archived from the original on 4 August 2009. Retrieved 27 April 2017.
- "Merck Announces First Phase Three Studies for PCV-15 (V114) Its Investigational Pneumococcal Disease Vaccine" (Press release). 17 April 2018. Retrieved 6 April 2020.
- Childhood Pneumococcal Disease - information on the disease and the Prevnar vaccine, from the Victoria State (Australia) government. Includes possible side effects.
- "Chapter 25: Pneumococcal" (PDF). Immunisation against infectious disease - 'The Green Book' (PDF). Department of Health (UK). 2006.
- "Recommended Child and Adolescent Immunization Schedule for ages 18 years or younger, United States, 2019". Centers for Disease Control and Prevention (CDC). 5 February 2019. Retrieved 3 November 2019.
- "CDC - ABCs: Surveillance Reports main page - Active Bacterial Core surveillance".
- Allen, Arthur (21 June 2007). "What if a vaccine makes room for a new strain of a disease?". Slate.com. Retrieved 27 April 2017.
- Whitney CG, Farley MM, Hadler J, et al. (May 2003). "Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine". The New England Journal of Medicine. 348 (18): 1737–46. doi:10.1056/NEJMoa022823. PMID 12724479.
- Poehling KA, Talbot TR, Griffin MR, et al. (April 2006). "Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine". JAMA: The Journal of the American Medical Association. 295 (14): 1668–74. doi:10.1001/jama.295.14.1668. PMID 16609088.
- Whitney CG, Pilishvili T, Farley MM, et al. (October 2006). "Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study". Lancet. 368 (9546): 1495–502. doi:10.1016/S0140-6736(06)69637-2. PMID 17071283.
- Millar EV, Watt JP, Bronsdon MA, et al. (2008). "Indirect effect of 7‐valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members". Clin Infect Dis. 47 (8): 989–996. doi:10.1086/591966. PMID 18781875.
- Siemieniuk, Reed A.C.; Gregson, Dan B.; Gill, M. John (November 2011). "The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study". BMC Infectious Diseases. 11: 314. doi:10.1186/1471-2334-11-314. PMC 3226630. PMID 22078162.
- Barocchi MA, Censini S, Rappuoli R (2007). "Vaccines in the era of genomics: the pneumococcal challenge". Vaccine. 25 (16): 2963–73. doi:10.1016/j.vaccine.2007.01.065. PMID 17324490.
- Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR (April 2007). "Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis". Lancet. 369 (9568): 1179–86. doi:10.1016/S0140-6736(07)60564-9. PMID 17416262.
- Tsai CJ, Griffin MR, Nuorti JP, Grijalva CG (June 2008). "Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States". Clinical Infectious Diseases. 46 (11): 1664–72. doi:10.1086/587897. PMC 4822508. PMID 18433334.
- O'Brien KL, Wolfson LJ, Watt JP, et al. (2009). "Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates". Lancet. 374 (9693): 893–902. doi:10.1016/S0140-6736(09)61204-6. PMID 19748398.
- "PneumoADIP - Need for PneumoADIP". Pneumoasdip.com. Retrieved 27 April 2017.
- Johns Hopkins Bloomberg School of Public Health, International Vaccine Access Center (2013). "VIMS Report: Global vaccine introduction" (PDF). Jhsph.edu. Retrieved 27 April 2017.
- "Wyeth Annual report". Phx.xorporate-ir.net. Archived from the original on 6 December 2007. Retrieved 27 April 2017.
Further reading
- Centers for Disease Control and Prevention (2015). "Chapter 17: Pneumococcal Disease". In Hamborsky J, Kroger A, Wolfe S (eds.). Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington, D.C.: Public Health Foundation.
External links
- "13-Valent Pneumococcal Vaccine". Drug Information Portal. U.S. National Library of Medicine.
- "Heptavalent pneumococcal conjugate vaccine". Drug Information Portal. U.S. National Library of Medicine.
- "Pneumococcal Conjugate (PCV13) Vaccine Information Statement". Centers for Disease Control and Prevention (CDC).