Precorrin-2 dehydrogenase
In enzymology, a precorrin-2 dehydrogenase (EC 1.3.1.76) is an enzyme that catalyzes the chemical reaction
- precorrin-2 + NAD+ sirohydrochlorin + NADH + H+
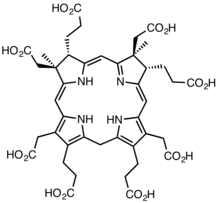 Precorrin-2 |
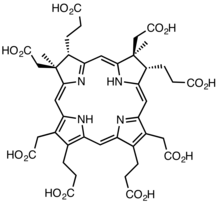 Sirohydrochlorin |
| precorrin-2 dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.3.1.76 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
The two substrates of this enzyme are precorrin-2 and NAD+; its three products are sirohydrochlorin, NADH, and H+.
This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-CH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is precorrin-2:NAD+ oxidoreductase. Other names in common use include Met8p, SirC, and CysG. This enzyme is part of the biosynthetic pathway to cobalamin (vitamin B12) in anaerobic bacteria and to Cofactor F430.
See Also
gollark: Also it's impossible to view it without JS enabled.
gollark: I would suspect you're overwriting the public key then loading some signed code, but the loadstring looks too short for a signature.
gollark: It does? Neat.
gollark: Why the `shell.run` *and* `loadstring` though?
gollark: Hmm, this is interesting and gives me some ideas.
References
- Warren MJ; Raux, E; Brindley, AA; Leech, HK; Wilson, KS; Hill, CP; Warren, MJ (2002). "The structure of Saccharomyces cerevisiae Met8p, a bifunctional dehydrogenase and ferrochelatase". EMBO J. 21 (9): 2068–75. doi:10.1093/emboj/21.9.2068. PMC 125995. PMID 11980703.
- Warren MJ, Raux E, Schubert HL, Escalante-Semerena JC (2002). "The biosynthesis of adenosylcobalamin (vitamin B12)". Nat. Prod. Rep. 19 (4): 390–412. doi:10.1039/b108967f. PMID 12195810.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.