Leaf-nosed bat
The New World leaf-nosed bats (Phyllostomidae) are found from southern North America to South America, specifically from Mexico to northern Argentina. They are ecologically the most varied and diverse family within the order Chiroptera. Most species are insectivorous, but the phyllostomid bats include within their number true predatory species and frugivores (subfamily Stenodermatinae and Carolliinae). For example, the spectral bat (Vampyrum spectrum), the largest bat in the Americas, eats vertebrate prey, including small, dove-sized birds. Members of this family have evolved to use food groups such as fruit, nectar, pollen, insects, frogs, other bats, and small vertebrates, and in the case of the vampire bats, even blood.
| Leaf-nosed bats | |
|---|---|
 | |
| Artibeus sp. | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Chiroptera |
| Superfamily: | Noctilionoidea |
| Family: | Phyllostomidae Gray, 1825 |
| Subfamilies | |
|
Brachyphyllinae | |
Both the scientific and common names derive from their often large, lance-shaped noses, greatly reduced in some of the nectar- and pollen-feeders. Because these bats echolocate nasally, this "nose-leaf" is thought to serve some role in modifying and directing the echolocation call. Similar nose leaves are found in some other groups of bats, most notably the Old World leaf-nosed bats.

New World leaf-nosed bats are usually brown, grey, or black, although five species are white. They range in size from 4.0 to 13.5 cm (1.6 to 5.3 in) in head-body length, and can weigh from 7 to 200 g (0.25 to 7.05 oz). Most roost in fairly small groups within caves, animal burrows, or hollow trees, although some species aggregate in colonies of several hundred individuals.[1] They do not hibernate, although some species have been reported to aestivate.[2][3]
Evolution
The Phyllostomidae, also known as New World leaf-nosed bats, are among the most ecologically diverse mammal families, displaying more morphological variation than any other mammalian family. This variation is measured by diversity in skull morphology and diet-related characteristics: Phyllostomidae consists of species that have evolved physical modifications for insectivory, frugivory, hematophagy, nectarivory, and omnivory.[4][5] The nose-leaf—a distinctive characteristic of the family—is thought to have evolved to reflect dietary and foraging behavior of different species of Phyllostomidae.[6] With an evolutionary history tracing back to the Oligocene, fossil and phylogenetic evidence suggests the family originated about 30 million years ago.[7] Leaf nosed bats evolved from Yangochiroptera and Miniopteridae with sister groups also evolving from this group. The Phyllostomidae consist of 55 genera and about 180 species.[5][8]
Description
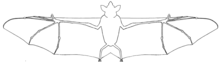
New World leaf-nosed bats are bilaterally symmetrical and endothermic mammals[9] characterized by an elaborate outgrowth of skin on their noses, called a nose-leaf, which is believed to aid in echolocation.[10] The nose-leaf can be adorned with a vertical leaf, a concave upward leaf, or multiple accessory leaves; varying by species.[11] Leaf-nosed bats lack a tail,[10] have triangular-shaped ears that can have pointed or rounded tips,[10] range in body size from 4 cm to 13.5 cm, and a wingspan up to 90 cm or more.[8]
Biology and ecology
Like other bats, leaf-nosed bats are nocturnal foragers that use echolocation to locate food sources, though the food sources vary between species.[12] Many bats in the family Phyllostomidae appear to have limited reliance on echolocation, likely because frugivorous bats do not need to quickly identify flying insects like many other bats.[6] Instead, species of leaf-nosed fruit bats appear to use scent to identify their preferred food sources.[13]
When they are not foraging, leaf-nosed bats roost in abandoned buildings, caves, and beneath folded leaves depending on the species. Nearly every roosting option present among bats is represented within this family, including species that prefer to roost alone, as well as species that roost with thousands of other individuals every day.[14][15]
Diet
The Phyllostomidae demonstrate the most diverse dietary habits of any family of bats across the globe.[16] Because of this, general dietary patterns are categorized for each species. Leaf-nosed bats generally specialize in a particular type of diet which leads to classification in one of these groups: frugivore, nectarivore, insectivore, omnivore, or haematophagous.[14] However, categorizations are based only on primary consumption habits, therefore observing species that occasionally consume food items outside of their particular classifications is not uncommon.[14] Usually, when leaf-nosed bats consume outside of their primary dietary categorization, it is to ensure sufficient intake of nutrients that their primary food source may not provide. For example, nectar and ripe fruits provide sufficient amounts of carbohydrates and water, but are lacking in protein and fat.[17] To meet basic nutritional requirements, leaf-nosed bats that primarily feed on fruit and nectar must also ensure sufficient protein and fat intake by consuming insects or leaves.[17]
Most leaf-nosed bats are classified as insectivores and feed on a variety of small insects. Certain species with this classification capture their prey either while in flight or from foliage in trees or on the ground. Carnivorous species feed on a variety of animals ranging from frogs to other bats. The Desmodontinae fall into this general carnivorous category, but are further distinguished by feeding exclusively on blood. In contrast, some species in this family feed on exclusively plants, gaining needed nutrients from fruits and leaves.[16]
Life cycle
Leaf-nosed bats are gonochoric (separate sexes) that partake in sexual copulation.[9] These bats can live for 20–30 years[18] and females become sexually active at two years of age.[19] Female ovulation occurs from October through September, after the female mates, the gestation period ranges from 8–9 months with an initial 3- to 5-month diapause period when the fetus growth is slowed; this diapause period is controlled by hormones.[19] The female gives birth to a single pup, which has open ears, open eyes,[18] and the first set of deciduous teeth,[20] and is fully furred at birth.[18]
Social systems
Among species that roost in groups, some evidence exists for a social hierarchy with higher-ranking individuals gaining access to preferred areas of the site.[21] Solitary roosting bats, though, live alone and maintain a strict fidelity to a single roosting site.[22] In some cases, males live alone or with harems, while females prefer to roost with other individuals and their pups.[23] In nearly every species that has been studied, mothers and pups maintain a social bond that lasts beyond nursing.[22] Apparently, young bats can learn food preferences from their mothers and when they are reluctant to leave the nest, mothers literally nudge the infants out of the roost.[24][13]
Range
New World leaf-nosed bats range from the United States, in southern Arizona and the West Indies to northern Argentina.[25][26] The family inhabits a diverse array of environments and habitats ranging from forests to deserts.[8]
Human impact
Species of New World leaf-nosed bats that make their homes in forested areas are greatly affected by agricultural intensification.[27] Specifically, it has been found that increased agricultural activity by humans causes negative conservation effects on these habitats and as a result reduces abundance and diversity of leaf-nosed bats that live there.[27] California leaf-nosed bats in particular are susceptible to human disruption. This species is known to create large roosts in closed mine shafts due to their potential to provide warmth and isolation.[28] When humans enter the shafts or rework old mines, this disrupts the roosts of the leaf-nosed bats and has the potential to be detrimental to the population as a whole.
Classification
The 192 described species within 56 genera are:
FAMILY PHYLLOSTOMIDAE
- Genus †Notonycteris[29]
- Subfamily: Brachyphyllinae
- Genus: Brachyphylla
- Cuban fruit-eating bat, B. nana
- Antillean fruit-eating bat, B. cavernarum
- Genus: Brachyphylla
- Subfamily: Carolliinae
- Genus: Carollia - short-tailed leaf-nosed bats
- Benkeith's short-tailed bat, C. benkeithi
- Silky short-tailed bat, 'C. brevicauda
- Chestnut short-tailed bat, C. castanea
- Colombian short-tailed bat, C. colombiana
- Manu short-tailed bat, C. manu
- Mono's short-tailed bat, C. monohernandezi
- Seba's short-tailed bat, C. perspicillata
- Sowell's short-tailed bat, C. sowelli
- Gray short-tailed bat, C. subrufa
- Genus: Rhinophylla
- Hairy little fruit bat, R. alethina
- Fischer's little fruit bat, R. fischerae
- Dwarf little fruit bat, R. pumilio
- Genus: Carollia - short-tailed leaf-nosed bats
- Subfamily: Desmodontinae - vampire bats
- Genus: Desmodus
- D. archaeodaptes, †
- Giant vampire bat, D. draculae†,[30][31]
- Cuban vampire bat, D. puntajudensis† [30][32]
- Common vampire bat, D. rotundus [33][34]
- Stock's vampire bat, D. stocki†,[30][35]
- Genus: Diaemus
- White-winged vampire bat, D. youngi
- Genus: Diphylla
- Hairy-legged vampire bat, D. ecaudata
- Genus: Desmodus
- Subfamily: Glossophaginae
- Tribe Glossophagini
- Genus: Anoura - Geoffroy's long-nosed bats
- Anoura aequatoris
- Cadena's tailless bat, A. cadenai
- Tailed tailless bat, A. caudifera
- Handley's tailless bat, A. cultrata
- Tube-lipped nectar bat, A. fistulata
- Geoffroy's tailless bat, A. geoffroyi
- Broad-toothed tailless bat, A. latidens
- Luis Manuel's tailless bat, A. luismanueli
- Anoura peruana
- Genus: Choeroniscus
- Godman's long-tailed bat, C. godmani
- Greater long-tailed bat, C. periosus
- Lesser long-tongued bat, C. minor
- Genus: Choeronycteris
- Mexican long-tongued bat (hog-nosed bat), C. mexicana
- Genus: Dryadonycteris
- Dryades bat, D. capixaba
- Genus: Glossophaga
- Commissaris's long-tongued bat, G. commissarisi
- Gray long-tongued bat, G. leachii
- Miller's long-tongued bat, G. longirostris
- Western long-tongued bat, G. morenoi
- Pallas's long-tongued bat, G. soricina
- Genus: Hylonycteris
- Underwood's long-tongued bat, H. underwoodi
- Genus: Leptonycteris - Saussure's long-nosed bats
- Southern long-nosed bat, L. curasoae
- Greater long-nosed bat or Mexican long-nosed bat, L. nivalis
- Lesser long-nosed bat or Mexican long-nosed bat, L. yerbabuenae
- Genus: Lichonycteris
- Dark long-tongued bat, L. obscura
- Genus: Monophyllus
- Insular single leaf bat, M. plethodon
- Leach's single leaf bat, M. redmani
- Genus: Musonycteris
- Banana bat (Colima long-nosed bat), M. harrisoni
- Genus: Scleronycteris
- Ega long-tongued bat, S. ega
- Genus: Anoura - Geoffroy's long-nosed bats
- Tribe Lonchophyllini
- Genus: Lionycteris
- Chestnut long-tongued bat, L. spurrelli
- Genus: Lonchophylla
- Bokermann's nectar bat, L. bokermanni
- Cadena's long-tongued bat, L. cadenai
- Chocoan long-tongued bat, L. chocoana
- Lonchophylla concava
- Dekeyser's nectar bat, L. dekeyseri
- Arched nectar bat, L. fornicata
- Handley's nectar bat, L. handleyi
- Western nectar bat, L. hesperia
- Goldman's nectar bat, L. mordax
- Orcés’s long-tongued bat, L. orcesi
- Lonchophylla orienticollina
- Patton's long-tongued bat, L. pattoni
- Lonchophylla peracchii
- Orange nectar bat, L. robusta
- Thomas's nectar bat, L. thomasi
- Genus: Platalina
- Long-snouted bat, P. genovensium
- Genus: Xeronycteris
- Vieira's long-tongued bat, X. vieirai
- Genus: Lionycteris
- Tribe Glossophagini
- Subfamily: Phyllonycterinae
- Genus: Erophylla - brown flower bats
- Brown flower bat, E. bombifrons
- Buffy flower bat, E. sezekorni
- Genus: Phyllonycteris - Jamaican flower bats
- Jamaican flower bat, P. aphylla
- Puerto Rican flower bat, P. major
- Cuban flower bat, P. poeyi
- Genus: Erophylla - brown flower bats
- Subfamily: Phyllostominae
- Tribe Micronycterini
- Genus: Glyphonycteris
- Behn's bat, G. behnii
- Davies's big-eared bat, G. daviesi
- Tricolored big-eared bat, G. sylvestris
- Genus: Lampronycteris
- Yellow-throated big-eared bat, L. brachyotis
- Genus: Macrotus - leaf-nosed bats
- California leaf-nosed bat, M. californicus
- Waterhouse's leaf-nosed bat, M. waterhousii
- Genus: Micronycteris - little big-eared bats
- Brosset's big-eared bat, M. brosseti
- Giovanni's big-eared bat, M. giovanniae
- Hairy big-eared bat, M. hirsuta
- Pirlot's big-eared bat, M. homezi
- Matses's big-eared bat, M. matses
- Little big-eared bat, M. megalotis
- Common big-eared bat, M. microtis
- White-bellied big-eared bat, M. minuta
- Sanborn's big-eared bat, M. sanborni
- Schmidts's big-eared bat, M. schmidtorum
- Genus: Neonycteris
- Least big-eared bat, N. pusilla
- Genus: Trinycteris
- Niceforo's big-eared bat, T. nicefori
- Genus: Glyphonycteris
- Tribe Vampyrini
- Genus: Chrotopterus
- Big-eared woolly bat, C. auritus
- Genus: Lophostoma
- Pygmy round-eared bat, L. brasiliense
- Carriker's round-eared bat, L. carrikeri
- Davis's round-eared bat, L. evotis
- Western round-eared bat, L. occidentalis
- Schultz's round-eared bat, L. schulzi
- White-throated round-eared bat, L. silvicolum
- Yasuni round-eared bat, L. yasuni
- Genus: Tonatia - round-eared bats
- Greater round-eared bat, T. bidens
- Stripe-headed round-eared bat, T. saurophila
- Genus: Trachops
- Fringe-lipped bat, T. cirrhosus
- Genus: Vampyrum
- Spectral bat, V. spectrum
- Genus: Chrotopterus
- Tribe Lonchorhinini
- Genus: Lonchorhina - sword-nosed bats
- Tomes's sword-nosed bat, L. aurita
- Fernandez's sword-nosed bat, L. fernandezi
- Northern sword-nosed bat, L. inusitata
- Marinkelle's sword-nosed bat, L. marinkellei
- Orinoco sword-nosed bat, L. orinocensis
- Genus: Macrophyllum
- Long-legged bat, M. macrophyllum
- Genus: Mimon - Gray's spear-nosed bats
- Golden bat, M. bennettii
- Cozumelan golden bat, M. cozumelae
- Striped hairy-nosed bat, M. crenulatum
- Koepcke's hairy-nosed bat, M. koepckeae
- Genus: Lonchorhina - sword-nosed bats
- Tribe Phyllostomatini
- Genus: Phylloderma - Peters's spear-nosed bat
- Pale-faced bat, P. stenops
- Genus: Phyllostomus - spear-nosed bats
- Pale spear-nosed bat, P. discolor
- Lesser spear-nosed bat, P. elongatus
- Greater spear-nosed bat, P. hastatus
- Guianan spear-nosed bat, P. latifolius
- Genus: Phylloderma - Peters's spear-nosed bat
- Tribe Micronycterini
- Subfamily: Stenodermatinae
- Genus: Ametrida
- Little white-shouldered bat, A. centurio
- Genus: Ardops
- Tree bat, A. nichollsi
- Genus: Ariteus
- Jamaican fig-eating bat, A. flavescens
- Genus: Artibeus - Neotropical fruit bats
- Subgenus: Artibeus
- Large fruit-eating bat, A. amplus
- Fringed fruit-eating bat, A. fimbriatus
- Fraternal fruit-eating bat, A. fraterculus
- Hairy fruit-eating bat, A. hirsutus
- Honduran fruit-eating bat, A. inopinatus
- Jamaican fruit bat, A. jamaicensis
- Great fruit-eating bat, A. lituratus
- Dark fruit-eating bat, A. obscurus
- Flat-faced fruit-eating bat, A. planirostris
- Artibeus schwartzi
- Subgenus: Dermanura
- Andersen's fruit-eating bat, A. anderseni
- Aztec fruit-eating bat, A. aztecus
- Bogota fruit-eating bat, A. bogotensis
- Gervais's fruit-eating bat, A. cinereus
- Silver fruit-eating bat, A. glaucus
- Gnome fruit-eating bat, A. gnomus
- Solitary fruit-eating bat, A. incomitatus
- Pygmy fruit-eating bat, A. phaeotis
- Rosenberg's fruit-eating bat, A. rosenbergi
- Toltec fruit-eating bat, A. toltecus
- Thomas's fruit-eating bat, A. watsoni
- Subgenus: Koopmania
- Brown fruit-eating bat, K. concolor
- Subgenus: Artibeus
- Genus: Centurio
- Wrinkle-faced bat, C. senex
- Genus: Chiroderma - big-eyed bats or white-lined bats
- Brazilian big-eyed bat, C. doriae
- Guadeloupe big-eyed bat, C. improvisum
- Salvin's big-eyed bat, C. salvini
- Little big-eyed bat, C. trinitatum
- Hairy big-eyed bat, C. villosum
- Genus: Ectophylla
- Honduran white bat, E. alba
- Genus: Enchisthenes
- Velvety fruit-eating bat, E. hartii
- Genus: Mesophylla
- MacConnell's bat, M. macconnelli
- Genus: Phyllops - falcate-winged bats
- Cuban fig-eating bat, P. falcatus
- Genus: Platyrrhinus
- Alberico's broad-nosed bat, P. albericoi
- Platyrrhinus aquilus
- Slender broad-nosed bat P. angustirostris
- Eldorado broad-nosed bat, P. (Vampyrops) aurarius
- Short-headed broad-nosed bat, P. (Vampyrops) brachycephalus
- Choco broad-nosed bat, P. chocoensis
- Thomas's broad-nosed bat, P. (Vampyrops) dorsalis
- Brown-bellied broad-nosed bat P. fusciventris
- Heller's broad-nosed bat, P. (Vampyrops) helleri
- Platyrrhinus incarum
- Buffy broad-nosed bat, P. (Vampyrops) infuscus
- Ismael's broad-nosed bat, P. ismaeli
- White-lined broad-nosed bat, P. (Vampyrops) lineatus
- Quechua broad-nosed bat, P. masu
- Matapalo broad-nosed bat, P. matapalensis
- Geoffroy's rayed bat, P. nigellus
- Platyrrhinus nitelinea
- Recife broad-nosed bat, P. (Vampyrops) recifinus
- Shadowy broad-nosed bat, P. umbratus
- Greater broad-nosed bat, P. (Vampyrops) vittatus
- Genus: Pygoderma
- Ipanema bat, P. bilabiatum
- Genus: Sphaeronycteris
- Visored bat, S. toxophyllum
- Genus: Stenoderma
- Red fruit bat, S. rufum
- Genus: Sturnira - yellow-shouldered bats or American epauleted bats
- Aratathomas's yellow-shouldered bat, 'S. aratathomasi
- Bidentate yellow-shouldered bat, S. bidens
- Bogota yellow-shouldered bat, S. bogotensis
- Hairy yellow-shouldered bat, S. erythromos
- Chocó yellow-shouldered bat, S. koopmanhilli
- little yellow-shouldered bat, S. lilium
- Highland yellow-shouldered bat, S. ludovici
- Louis's yellow-shouldered bat, S. luisi
- Greater yellow-shouldered bat, S. magna
- Mistratoan yellow-shouldered bat, S. mistratensis
- Talamancan yellow-shouldered bat, S. mordax
- Lesser yellow-shouldered bat, S. nana
- Tschudi's yellow-shouldered bat, S. oporaphilum
- Sturnira perla Jarrin-V & Kunz, 2011[36]
- Soriano's yellow-shouldered bat, S. sorianoi
- Thomas's yellow-shouldered bat, S. thomasi
- Tilda's yellow-shouldered bat, S. tildae
- Genus: Uroderma - tent-building bats
- Tent-making bat, U. bilobatum
- Brown tent-making bat, U. magnirostrum
- Genus: Vampyressa - yellow-eared bats
- Vampyressa elisabethae
- Melissa's yellow-eared bat, V. melissa
- Southern little yellow-eared bat, V. pusilla
- Vampyressa sinchi
- Northern little yellow-eared bat, V. thyone
- Genus: Vampyriscus
- Bidentate yellow-eared bat, V. bidens
- Brock's yellow-eared bat, V. brocki
- Striped yellow-eared bat, V. nymphaea
- Genus: Vampyrodes
- Great stripe-faced bat, V. caraccioli
- Genus: Ametrida
References
| Wikimedia Commons has media related to Phyllostomidae. |
| Wikispecies has information related to Phyllostomidae |
- Garbino, Guilherme S. T.; Tavares, Valéria da Cunha (2018). "Roosting ecology of Stenodermatinae bats (Phyllostomidae): evolution of foliage roosting and correlated phenotypes". Mammal Review. 48 (2): 75–89. doi:10.1111/mam.12114. ISSN 1365-2907.
- Macdonald, D., ed. (1984). The Encyclopedia of Mammals. New York: Facts on File. pp. 805. ISBN 978-0-87196-871-5.
- Wetterer, Andrea L.; et al. (2000). "Phylogeny of Phyllostomid Bats (Mammalia: Chiroptera): Data from Diverse Morphological Systems, Sex Chromosomes, and Restriction Sites". Bulletin of the American Museum of Natural History. 248 (1): 1–200. doi:10.1206/0003-0090(2000)248<0001:POPBMC>2.0.CO;2. hdl:2246/1595.
- j. Baker, Robert; r. Hoofer, Steven; Porter, Calvin; Van Den Bussche, Ronald (2003-12-19). "Diversification among New World leaf-nosed bats: An evolutionary hypothesis and classification inferred from digenomic congruence of DNA sequence". Occasional Papers, Museum of Texas Tech University. 230: 1–32. Retrieved 2018-10-31.
- Dumont, Elizabeth R.; Dávalos, Liliana M.; Goldberg, Aaron; Santana, Sharlene E.; Rex, Katja; Voigt, Christian C. (2012-05-07). "Morphological innovation, diversification and invasion of a new adaptive zone". Proc. R. Soc. B. 279 (1734): 1797–1805. doi:10.1098/rspb.2011.2005. ISSN 0962-8452. PMC 3297451. PMID 22113035.
- Bogdanowicz, W.; Csada, R. D.; Fenton, M. B. (1997-08-22). "Structure of Noseleaf, Echolocation, and Foraging Behavior in the Phyllostomidae (Chiroptera)". Journal of Mammalogy. 78 (3): 942–953. doi:10.2307/1382954. ISSN 1545-1542. JSTOR 1382954.
- Rojas, Danny; Warsi, Omar M.; Dávalos, Liliana M. (2016-02-10). "Bats (Chiroptera: Noctilionoidea) Challenge a Recent Origin of Extant Neotropical Diversity". Systematic Biology. 65 (3): 432–448. doi:10.1093/sysbio/syw011. ISSN 1063-5157. PMID 26865275.
- "Phyllostomidae | mammal family". Encyclopedia Britannica. Retrieved 2018-10-31.
- "Phyllostomidae — Overview New World Leaf-nosed Bats". Encyclopedia of Life.
- "Griffin's leaf-nosed bat videos, photos and facts - Hipposideros griffini". Arkive. Archived from the original on 2013-03-02. Retrieved 2018-11-01.
- "Leaf-nosed bat | mammal". Encyclopedia Britannica. Retrieved 2018-11-01.
- Fenton, M.B. (1990). "The foraging behaviour and ecology of animal-eating bats". Canadian Journal of Zoology. 68 (3): 411–422. doi:10.1139/z90-061.
- Ganesh, A.; Mukilan, M.; Marimuthu, G.; Rajan, K.E. (June 2016). "A Novel Food Preference in the Greater Short-Nosed Fruit Bat, Cynopterus sphinx: Mother-Pup Interaction a Strategy for Learning". Acta Chiropterologica. 18 (1): 193–198. doi:10.3161/15081109ACC2016.18.1.009.
- Kries, Kelly; Barros, Marilia; Duytschaever, Gwen (30 July 2018). "Colour vision variation in leaf‐nosed bats (Phyllostomidae): Links to cave roosting and dietary specialization". Molecular Ecology. 27 (18). doi:10.1111/mec.14818.
- Rodriguez-Herrera, Bernal; Rodriguez, Melissa; Otarola, Mauricio Fernandez (2018). "Ecological Networks between Tent-Roosting Bats (Phyllostomidae: Stenodermatinae) and the Plants Used in a Neotropical Rainforest". Acta Chiropterologica. 20 (1): 139–145. doi:10.3161/15081109ACC2018.20.1.010.
- Korine, C.; Kalko, E. K. V. (2005). "Fruit detection and discrimination by small fruit-eating bats (Phyllostomidae): echolocation call design and olfaction". Behavioral Ecology and Sociobiology. 59 (1): 12–23. doi:10.1007/s00265-005-0003-1.
- Elangovan, V., Marimuthu, G., Kunz, T.H. Temporal patterns of resource use by the short-nosed fruit bat, Cynopterus sphinx (Megachiroptera: Pteropodidae) (2001) Journal of Mammalogy, 82 (1), pp. 161-165.
- "California Leaf-nosed bat Fact Sheet". www.desertmuseum.org. Retrieved 2018-11-01.
- "California Leaf-Nosed Bat (Macrotus californicus)" (PDF). DUDEK ICF International.
- Jin, Long-ru; Lin, Ai-qing; Sun, Ke-ping; Liu, Ying; Feng, Jiang (2010-11-05). "Postnatal development of morphological features and vocalization in the pomona leaf-nosed bat Hipposideros pomona". Acta Theriologica. 56 (1): 13–22. doi:10.1007/s13364-010-0011-z. ISSN 0001-7051.
- Selvanayagam, P.F.L.; Marimuthu, G. (April 1984). "Spatial organization of roosting in the insectivorous tropical bat Hipposideros speoris". Behavioural Processes. 9 (2–3): 113–121. doi:10.1016/0376-6357(84)90033-0. PMID 24896509.
- Dwyer, P.D. (1970). "Social organization in the bat Myotis adversus". Science. 168 (3934): 1006–1008. Bibcode:1970Sci...168.1006D. doi:10.1126/science.168.3934.1006. PMID 5441022.
- York, H.A.; Foster, P.F.; Jones, M.F. (1 May 2008). "Observations of cavity-roosting behavior in Costa Rican Lophostoma brasiliense (Chiroptera: Phyllostomidae)". Mammalian Biology. 73 (3): 230–232. doi:10.1016/j.mambio.2007.02.008.
- Lallensack, Rachael. "Mama Bats Literally Nudge Their Babies Out of the Nest". Smithsonian.com. Smithsonian Institution.
- Rossoni, Daniela M.; Assis, Ana Paula A.; Giannini, Norberto P.; Marroig, Gabriel (2017-09-11). "Intense natural selection preceded the invasion of new adaptive zones during the radiation of New World leaf-nosed bats". Scientific Reports. 7 (1): 11076. Bibcode:2017NatSR...711076R. doi:10.1038/s41598-017-08989-6. ISSN 2045-2322. PMC 5593990. PMID 28894101.
- Villalobos, Fabricio; Arita, Héctor T. (2009-11-27). "The diversity field of New World leaf-nosed bats (Phyllostomidae)". Global Ecology and Biogeography. 19 (2): 200–211. doi:10.1111/j.1466-8238.2009.00503.x. ISSN 1466-822X.
- Williams‐Guillén, K., & Perfecto, I. (2010). Effects of Agricultural Intensification on the Assemblage of Leaf-Nosed Bats (Phyllostomidae) in a Coffee Landscape in Chiapas, Mexico. Biotropica, 42(5), 605–613.
- Kunz, T.H. (1982). Roosting Ecology of Bats. Ecology of Bats pp. 1-55.
- Notonycteris at Fossilworks.org
- Turvey, S.T. (2009). Holocene mammal extinctions. In: Turvey, S.T. (editor) (2009). Holocene Extinctions. Oxford University Press, Oxford, UK.
- Turvey, S. 2008. Desmodus draculae. Archived 2016-03-05 at the Wayback Machine The IUCN Red List of Threatened Species. Downloaded on 02 March 2016.
- Suárez, W (2005). "Taxonomic status of the Cuban vampire bat (Chiroptera: Phyllostomidae: Desmodontinae: Desmodus)" (PDF). Caribbean Journal of Science. 41 (4): 761–767.
- Don E. Wilson & DeeAnn M. Reeder (editors). 2005. Mammal Species of the World. A Taxonomic and Geographic Reference (3rd ed), Johns Hopkins University Press, 2,142 pp.
- Barquez, R., Perez, S., Miller, B. & Diaz, M. 2015. Desmodus rotundus. The IUCN Red List of Threatened Species. Downloaded on 02 March 2016.
- Knox Jones JR, J. (1958). Pleistocene Bats from San Josecito Cave, Nuevo Leon, Mexico. University of Kansas Publications, Museum of Natural History, Volume 9, No. 14, pp. 389-396, December 19, 1958. (Available online)
- Jarrin-V, P.; Kunz, T. H. (2011). "A new species of Sturnira (Chiroptera: Phyllostomidae) from the Choco forest of Ecuador" (PDF). Zootaxa. 2755: 1–35. doi:10.11646/zootaxa.2755.1.1.