NDUFS8
NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, mitochondrial also known as NADH-ubiquinone oxidoreductase 23 kDa subunit, Complex I-23kD (CI-23kD), or TYKY subunit is an enzyme that in humans is encoded by the NDUFS8 gene.[5][6][7][8] The NDUFS8 protein is a subunit of NADH dehydrogenase (ubiquinone) also known as Complex I, which is located in the mitochondrial inner membrane and is the largest of the five complexes of the electron transport chain.[9] Mutations in this gene have been associated with Leigh syndrome.[7]
Structure
NDUFS8 is located on the q arm of chromosome 11 in position 13.2.[7] The NDUFS8 gene produces a 23.7 kDa protein composed of 210 amino acids.[10][11] The encoded protein, TYKY, contains two 4Fe4S ferredoxin consensus patterns which are believed to be iron-sulfur cluster N-2 binding sites.[12] Studies of other subunits of Complex I have suggested that the subunits TYKY, PSST, 49 kDa, ND1, and ND5 interact with iron-sulfur clusters to form the catalytic core of NADH dehydrogenase (ubiquinone).[13]
Function
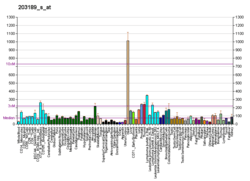
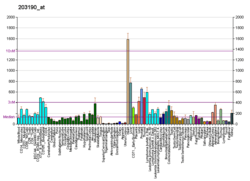
This gene encodes a subunit of mitochondrial NADH:ubiquinone oxidoreductase, or Complex I, a multimeric enzyme of the respiratory chain responsible for NADH oxidation, ubiquinone reduction, and the ejection of protons from mitochondria. The encoded protein is involved in the binding of two of the six to eight iron-sulfur clusters of Complex I and, as such, is required in the electron transfer process.[7]
Clinical significance
Mutations in NDUFS8 have been associated with mitochondrial diseases, which can cause any one of a clinically heterogeneous group of disorders arising from dysfunction of the mitochondrial respiratory chain. The phenotypic spectrum ranges from isolated diseases affecting single organs to severe multisystem disorders. Common clinical features include ptosis, external ophthalmoplegia, proximal myopathy and exercise intolerance, cardiomyopathy, sensorineural deafness, optic atrophy, pigmentary retinopathy, encephalopathy, seizures, stroke-like episodes, ataxia, spasticity and lactic acidosis. Mitochondrial disorders can be caused by mutations of mitochondrial DNA or nuclear DNA that either affect oxidative phosphorylation proteins directly, or affect respiratory chain function by impacting the production of the complex machinery needed to run this process.[8]
NDUFS8 mutations have also been associated with Leigh syndrome. Leigh syndrome is an early-onset progressive neurodegenerative disorder characterized by the presence of focal, bilateral lesions in one or more areas of the central nervous system including the brainstem, thalamus, basal ganglia, cerebellum and spinal cord. Clinical features depend on which areas of the central nervous system are involved and include subacute onset of psychomotor retardation, hypotonia, ataxia, weakness, vision loss, eye movement abnormalities, seizures, dysphagia and lactic acidosis.[8] One case report of a pathogenic mutation in NDUFS8 found that it resulted in complex I mitochondrial deficiency and a diagnosis of Leigh syndrome. The patient’s symptoms included apnea, cyanosis, hypercarbia, hypotonia, brisk tendon reflexes, ankle clonus, and erratic seizures. Further analysis revealed increased lactate, cerebral lesions, and a hypertrophic obstructive cardiomyopathy.[12]
Interactions
In addition to co-subunits for complex I, NDUFS8 has protein-protein interactions with MLH1 and GEM.[14][15]
References
- GRCh38: Ensembl release 89: ENSG00000110717 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000059734 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- de Sury R, Martinez P, Procaccio V, Lunardi J, Issartel JP (July 1998). "Genomic structure of the human NDUFS8 gene coding for the iron-sulfur TYKY subunit of the mitochondrial NADH:ubiquinone oxidoreductase". Gene. 215 (1): 1–10. doi:10.1016/S0378-1119(98)00275-3. PMID 9666055.
- Procaccio V, Depetris D, Soularue P, Mattei MG, Lunardi J, Issartel JP (March 1997). "cDNA sequence and chromosomal localization of the NDUFS8 human gene coding for the 23 kDa subunit of the mitochondrial complex I". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1351 (1–2): 37–41. doi:10.1016/s0167-4781(97)00020-1. PMID 9116042.
- "Entrez Gene: NDUFS8 NADH dehydrogenase (ubiquinone) Fe-S protein 8, 23kDa (NADH-coenzyme Q reductase)".
- "NDUFS8 - NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, mitochondrial precursor - Homo sapiens (Human) - NDUFS8 gene & protein". www.uniprot.org. Retrieved 2018-07-19.
- Donald Voet; Judith G. Voet; Charlotte W. Pratt (2013). "18". Fundamentals of biochemistry : life at the molecular level (4th ed.). Hoboken, NJ: Wiley. pp. 581–620. ISBN 9780470547847.
- Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, Deng N, Kim AK, Choi JH, Zelaya I, Liem D, Meyer D, Odeberg J, Fang C, Lu HJ, Xu T, Weiss J, Duan H, Uhlen M, Yates JR, Apweiler R, Ge J, Hermjakob H, Ping P (October 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- Yao, Daniel. "Cardiac Organellar Protein Atlas Knowledgebase (COPaKB) —— Protein Information". amino.heartproteome.org. Retrieved 2018-07-20.
- Loeffen J, Smeitink J, Triepels R, Smeets R, Schuelke M, Sengers R, Trijbels F, Hamel B, Mullaart R, van den Heuvel L (December 1998). "The first nuclear-encoded complex I mutation in a patient with Leigh syndrome". American Journal of Human Genetics. 63 (6): 1598–608. doi:10.1086/302154. PMC 1377631. PMID 9837812.
- Schuler, Franz; Casida, John E. (2001-07-02). "Functional coupling of PSST and ND1 subunits in NADH:ubiquinone oxidoreductase established by photoaffinity labeling". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1506 (1): 79–87. doi:10.1016/S0005-2728(01)00183-9. ISSN 0005-2728. PMID 11418099.
- "Cytoskeletal scaffolding proteins interact with Lynch-Syndrome associated mismatch repair protein MLH1". IntAct.
- "Protein interaction data set highlighted with human Ras-MAPK/PI3K signaling pathways". IntAct.
Further reading
- Procaccio V, Wallace DC (May 2004). "Late-onset Leigh syndrome in a patient with mitochondrial complex I NDUFS8 mutations". Neurology. 62 (10): 1899–901. doi:10.1212/01.wnl.0000125251.56131.65. PMC 2821060. PMID 15159508.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Dunbar DR, Shibasaki Y, Dobbie L, Andersson B, Brookes AJ (1997). "In situ hybridisation mapping of genomic clones for five human respiratory chain complex I genes". Cytogenetics and Cell Genetics. 78 (1): 21–4. doi:10.1159/000134618. PMID 9345899.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Triepels RH, Hanson BJ, van den Heuvel LP, Sundell L, Marusich MF, Smeitink JA, Capaldi RA (March 2001). "Human complex I defects can be resolved by monoclonal antibody analysis into distinct subunit assembly patterns". The Journal of Biological Chemistry. 276 (12): 8892–7. doi:10.1074/jbc.M009903200. PMID 11112787.
- Lescuyer P, Martinez P, Lunardi J (March 2002). "YY1 and Sp1 activate transcription of the human NDUFS8 gene encoding the mitochondrial complex I TYKY subunit". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1574 (2): 164–74. doi:10.1016/s0167-4781(01)00377-3. PMID 11955626.
- Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA (September 2003). "Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry". The Journal of Biological Chemistry. 278 (39): 37223–30. doi:10.1074/jbc.M305694200. PMID 12857734.
- Ugalde C, Janssen RJ, van den Heuvel LP, Smeitink JA, Nijtmans LG (March 2004). "Differences in assembly or stability of complex I and other mitochondrial OXPHOS complexes in inherited complex I deficiency". Human Molecular Genetics. 13 (6): 659–67. doi:10.1093/hmg/ddh071. PMID 14749350.
- Bourges I, Ramus C, Mousson de Camaret B, Beugnot R, Remacle C, Cardol P, Hofhaus G, Issartel JP (November 2004). "Structural organization of mitochondrial human complex I: role of the ND4 and ND5 mitochondria-encoded subunits and interaction with prohibitin". The Biochemical Journal. 383 (Pt. 3): 491–9. doi:10.1042/BJ20040256. PMC 1133742. PMID 15250827.
External links
- Overview of all the structural information available in the PDB for UniProt: O00217 (Human NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, mitochondrial) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: Q8K3J1 (Mouse NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, mitochondrial) at the PDBe-KB.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.