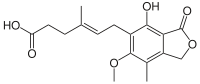
Mycophenolic acid
Mycophenolic acid (MPA) is an immunosuppressant medication used to prevent rejection following organ transplantation and to treat Crohn's disease.[4] Specifically it is used following kidney, heart, and liver transplantation.[4] It can be given by mouth or by injection into a vein.[4] It comes as mycophenolate sodium and mycophenolate mofetil.[4]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌmaɪkoʊfɪˈnɒlɪk/ |
| Trade names | CellCept, Myfortic, others |
| Other names | Mycophenolate sodium, mycophenolate mofetil |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601081 |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth, intravenous[2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 72% (sodium), 94% (mofetil)[3] |
| Protein binding | 82–97%[3] |
| Metabolism | Liver[3] |
| Elimination half-life | 17.9±6.5 hours[3] |
| Excretion | Urine (93%), faeces (6%)[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.912 |
| Chemical and physical data | |
| Formula | C17H20O6 |
| Molar mass | 320.341 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Common side effects include nausea, infections, and diarrhea.[4] Other serious side effects include an increased risk of cancer, progressive multifocal leukoencephalopathy, anemia, and gastrointestinal bleeding.[4] Use during pregnancy may harm the baby.[4] It works by blocking inosine monophosphate dehydrogenase (IMPDH), which is needed by lymphocytes to make guanosine.[4]
Mycophenolic acid was initially discovered by Italian Bartolomeo Gosio in 1893.[5][6] It was rediscovered in 1945 and 1968.[6] It was approved for medical use in the United States in 1995 following the discovery of its immunosuppressive properties in the 1990s.[4][5] It is available as a generic medication.[7] In 2017, it was the 254th most commonly prescribed medication in the United States, with more than one million prescriptions.[8][9]
Medical uses
Organ transplant
Mycophenolate is used for the prevention of organ transplant rejection. Mycophenolate mofetil is indicated for the prevention of organ transplant rejection in adults and kidney transplantation rejection in children over 2 years; whereas mycophenolate sodium is indicated for the prevention of kidney transplant rejection in adults. Mycophenolate sodium has also been used for the prevention of rejection in liver, heart, or lung transplants in children older than two years.[10]
Autoimmune disease
Mycophenolate is increasingly utilized as a steroid sparing treatment in autoimmune diseases and similar immune-mediated disorders including Behçet's disease, pemphigus vulgaris, immunoglobulin A nephropathy, small vessel vasculitides, and psoriasis.[11] It is also used for retroperitoneal fibrosis along with a number of other medications.[12] Specifically it has also be used for psoriasis not treatable by other methods.[13]
Its increasing application in treating lupus nephritis has demonstrated more frequent complete response and less frequent complications[11] compared to cyclophosphamide bolus therapy, a regimen with risk of bone marrow suppression, infertility, and malignancy.[14] Further work addressing maintenance therapy demonstrated mycophenolate superior to cyclophosphamide, again in terms of response and side-effects.[14] Walsh proposed that mycophenolate should be considered as a first-line induction therapy for treatment of lupus nephritis in people without kidney dysfunction.[15]
Comparison to other agents
Compared with azathioprine it has higher incidence of diarrhea, and no difference in risk of any of the other side effects.[16] Mycophenolic acid is 15 times more expensive than azathioprine.[17] The exact role of mycophenolate vs azathioprine has yet to be conclusively established.
Adverse effects
Common adverse drug reactions (≥1% of people) include diarrhea, nausea, vomiting, joint pain; infections, leukopenia, or anemia reflect the immunosuppressive and myelosuppressive nature of the drug. Mycophenolate sodium is also commonly associated with fatigue, headache, cough and/or breathing issues. Intravenous (IV) administration of mycophenolate mofetil is also commonly associated with thrombophlebitis and thrombosis. Infrequent adverse effects (0.1–1% of people) include esophagitis, gastritis, gastrointestinal tract hemorrhage, and/or invasive cytomegalovirus (CMV) infection.[10] More rarely, pulmonary fibrosis or various neoplasia occur: melanoma, lymphoma, other malignancies having an occurrences of 1 in 20 to 1 in 200, depending on the type, with neoplasia in the skin being the most common site.[18][19] Several cases of pure red cell aplasia (PRCA) have also been reported.[20]
The U.S. Food and Drug Administration (FDA) issued an alert that people are at increased risk of opportunistic infections, such as activation of latent viral infections, including shingles, other herpes infections, cytomegalovirus, and BK virus associated nephropathy. In addition the FDA is investigating 16 people that developed a rare neurological disease while taking the drug. This is a viral infection known as progressive multifocal leukoencephalopathy; it attacks the brain and is usually fatal.[21]
MMF and EC-MPS appear to be equal in benefits and safety.[22]
Pregnancy
Mycophenolic acid is associated with miscarriage and congenital malformations when used during pregnancy, and should be avoided whenever possible by women trying to get pregnant.[23][24]
Blood tests
Among the most common effects of this drug is increased blood cholesterol levels. Other changes in blood chemistry such as hypomagnesemia, hypocalcemia, hyperkalemia, and an increase in blood urea nitrogen (BUN) can occur.[2][25]
Mechanism of action
Purines (including the nucleotides guanosine and adenosine) can either be synthesized de novo using ribose 5-phosphate or they can be salvaged from free nucleotides. Mycophenolic acid is potent, reversible, non-competitive inhibitor of inosine-5′-monophosphate dehydrogenase (IMPDH), an enzyme essential to the de novo synthesis of guanosine-5'-monophosphate (GMP) from inosine-5'-monophosphate (IMP).[26] IMPDH inhibition particularly affects lymphocytes since they rely almost exclusively on de novo purine synthesis.[27] In contrast, many other cell types use both pathways, and some cells, such as terminally differentiated neurons, depend completely on purine nucleotide salvage.[28] Thus, use of mycophenolic acid leads to a relatively selective inhibition of DNA replication in T cells and B cells.
Pharmacology
Mycophenolate can be derived from the fungi Penicillium stoloniferum, P. brevicompactum and P. echinulatum.[29] Mycophenolate mofetil is metabolised in the liver to the active moiety mycophenolic acid. It reversibly inhibits inosine monophosphate dehydrogenase,[30] the enzyme that controls the rate of synthesis of guanine monophosphate in the de novo pathway of purine synthesis used in the proliferation of B and T lymphocytes.[31] Other cells recover purines via a separate salvage pathway and are thus able to escape the effect.[2]
Mycophenolate is potent and can, in many contexts, be used in place of the older anti-proliferative azathioprine.[32] It is usually used as part of a three-compound regimen of immunosuppressants, also including a calcineurin inhibitor (ciclosporin or tacrolimus) and a glucocorticoid (e.g. dexamethasone or prednisone).[33]
Chemistry
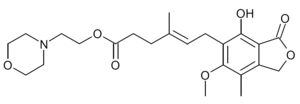
Mycophenolate mofetil is the morpholino ethyl ester of mycophenolic acid; the ester masks the carboxyl group.[34] Mycophenolate mofetil is reported to have a pKa values of 5.6 for the morpholino moiety and 8.5 for the phenolic group.
History
Mycophenolic acid was discovered by Italian medical scientist Bartolomeo Gosio. Gosio collected a fungus from spoiled corn and named it Penicillium glaucum. (The species is now called P. brevicompactum.) In 1893 he found that the fungus had antibacterial activity. In 1896 he isolated crystals of the compound, which he successfully demonstrated as the active antibacterial compound against the anthrax bacterium.[13] This was the first antibiotic that was isolated in pure and crystalline form. But the discovery was forgotten.[35] It was rediscovered by two American scientists C.L. Alsberg and O.M. Black in 1912, and gave the name mycophenolic acid. The compound was eventually demonstrated to have antiviral, antifungal, antibacterial, anticancer, and antipsoriasis activities.[36] Although it is not commercialised as antibiotic due to its adverse effects, its modified compound (ester derivative) is an approved immunosuppressant drug in kidney, heart, and liver transplantations, and is marketed under the brands CellCept (mycophenolate mofetil by Roche) and Myfortic (mycophenolate sodium by Novartis).[37]
Cellcept was developed by a South African geneticist Anthony Allison and his wife Elsie M. Eugui. In the 1970s while working at the Medical Research Council, Allison investigated the biochemical causes of immunune deficiency in children. He discovered the metabolic pathway involving an enzyme, inosine monophosphate dehydrogenase, which is responsible for undesirable immune response in autoimmune diseases, as well as for immune rejection in organ transplantation. He conceived an idea that if a molecule that could block the enzyme is discovered, then, it would become an immunosuppressive drug that could be used for autoimmune diseases and in organ transplantation. In 1981 he decided to go for drug discovery and approached several pharmaceutical companies, which turned him down one by one as he had no primary knowledge on drug research. However, Syntex liked his plans and asked him to join the company with his wife.[38] He became vice president for the research. In one of their experiments the Allisons used an antibacterial compound, mycophenolate mofetil, which was abandoned in clinical use due to its adverse effects. They discovered that the compound had immunosuppressive activity.[39][40] They synthesised a chemical variant for increased activity and reduced adverse effects.[41][42][43][44][45] They subsequently demonstrated that it was useful in organ transplantation in experimental rats.[46][47] After successful clinical trials,[48] the compound was approved for use in kidney transplant by the U.S. Food and Drug Administration on 3 May 1995,[49] and was sold under the brand name CellCept.[50][51] It was approved for use in the European Union in February 1996.[52]
Names
It was initially introduced as the prodrug mycophenolate mofetil (MMF, trade name CellCept) to improve oral bioavailability. The salt mycophenolate sodium has also been introduced. Enteric-coated mycophenolate sodium (EC-MPS) is an alternative MPA formulation.
MMF and EC-MPS appear to be equal in benefits and safety.[22]
Research
Mycophenolate mofetil is beginning to be used in the management of auto-immune disorders such as idiopathic thrombocytopenic purpura (ITP), systemic lupus erythematosus (SLE), scleroderma (systemic sclerosis or SSc), and pemphigus vulgaris (PV) with success for some patients.[53]
It is also currently being used as a long-term therapy for maintaining remission of granulomatosis with polyangiitis, though thus far, studies have found it inferior to azathioprine. A combination of mycophenolate and ribavirin has been found to stop infection by and replication of dengue virus in vitro.[54][55] It has also shown promising antiviral activity against MERS, especially in combination with interferon.[56]
Preliminary data suggests that mycophenolate mofetil might have benefits in people with multiple sclerosis. However the evidence is insufficient to determine the effects as an add‐on therapy for interferon beta-1a in people with RRMS.[57]
References
- "Mycophenolate mofetil (CellCept) Use During Pregnancy". Drugs.com. 24 January 2020. Retrieved 6 April 2020.
- Jasek W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 1484–95. ISBN 978-3-85200-181-4.
- "CellCept" (PDF). TGA eBusiness Services. Roche Products Pty Limited. 13 December 2012. Retrieved 25 February 2014.
- "Mycophenolate Monograph for Professionals". Drugs.com. Retrieved 28 October 2019.
- Schiff ER, Maddrey WC, Sorrell MF (2011). Schiff's Diseases of the Liver. John Wiley & Sons. p. PT3219. ISBN 978-1-119-95048-6.
- Laskin AI, Bennett JW, Gadd GM (2001). Advances in Applied Microbiology. Gulf Professional Publishing. p. 236. ISBN 978-0-12-002648-7.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 826–827. ISBN 978-0-85711-338-2.
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Mycophenolate Mofetil - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- Rossi S, ed. (2006). Australian Medicines Handbook. Adelaide: Australian Medicines Handbook. ISBN 978-0-9757919-2-9.
- Moore RA, Derry S (2006). "Systematic review and meta-analysis of randomised trials and cohort studies of mycophenolate mofetil in lupus nephritis". Arthritis Research & Therapy. 8 (6): R182. doi:10.1186/ar2093. PMC 1794528. PMID 17163990.
- Harber M (2014). Harber M (ed.). Practical nephrology. p. 449. ISBN 978-1-4471-5547-8.
- Kitchin JE, Pomeranz MK, Pak G, Washenik K, Shupack JL (September 1997). "Rediscovering mycophenolic acid: a review of its mechanism, side effects, and potential uses". Journal of the American Academy of Dermatology. 37 (3 Pt 1): 445–9. doi:10.1016/S0190-9622(97)70147-6. PMID 9308561.
- D'Cruz DP, Khamashta MA, Hughes GR (February 2007). "Systemic lupus erythematosus". Lancet. 369 (9561): 587–96. CiteSeerX 10.1.1.1008.5428. doi:10.1016/S0140-6736(07)60279-7. PMID 17307106.
- Walsh M, James M, Jayne D, Tonelli M, Manns BJ, Hemmelgarn BR (September 2007). "Mycophenolate mofetil for induction therapy of lupus nephritis: a systematic review and meta-analysis". Clinical Journal of the American Society of Nephrology. 2 (5): 968–75. doi:10.2215/CJN.01200307. PMID 17702723.
- Knight SR, Russell NK, Barcena L, Morris PJ (March 2009). "Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review". Transplantation. 87 (6): 785–94. doi:10.1097/TP.0b013e3181952623. PMID 19300178.
- Remuzzi G, Lesti M, Gotti E, Ganeva M, Dimitrov BD, Ene-Iordache B, et al. (2004). "Mycophenolate mofetil versus azathioprine for prevention of acute rejection in renal transplantation (MYSS): a randomised trial". Lancet. 364 (9433): 503–12. doi:10.1016/S0140-6736(04)16808-6. PMID 15302193.
- "CellCept, Myfortic (mycophenolate) dosing, indications, interactions, adverse effects, and more". reference.medscape.com.
- Publications, BNF. "Homepage - BNF Publications". www.bnf.org.
- "CellCept (mycophenolate mofetil) August 2009". U.S. Food and Drug Administration. August 14, 2009. Retrieved 2009-08-21.
- "CellCept (mycophenolate mofetil) August 2009". U.S. Food and Drug Administration. August 14, 2009. Retrieved 2009-08-21.
- van Gelder T, Hesselink DA (May 2015). "Mycophenolate revisited". Transplant International. 28 (5): 508–15. doi:10.1111/tri.12554. PMID 25758949.
- "FDA Issues Second CellCept Warning". newsinferno.com. 2008-05-18. Retrieved 2010-10-26.
- "MedWatch Safety Alerts for Human Medical Products". fda.gov. May 2008. Retrieved 2010-10-26.
- Drugs.com: Mycophenolic acid Side Effects
- Pharmacology North American Edition. Lippincott Williams & Wilkins. 2014. p. 625. ISBN 978-1-4511-9177-6.
- Parnham FP, Nijkamp MJ, eds. (2005). Principles of immunopharmacology (2nd rev. and extended ed.). Basel: Birkhèauser Verlag. p. 453. ISBN 978-3764358044.
- Fu R, Ceballos-Picot I, Torres RJ, Larovere LE, Yamada Y, Nguyen KV, et al. (May 2014). "Genotype-phenotype correlations in neurogenetics: Lesch-Nyhan disease as a model disorder". Brain. 137 (Pt 5): 1282–303. doi:10.1093/brain/awt202. PMC 3999711. PMID 23975452.
- Anderson HA, Bracewell JM, Fraser AR, Jones D, Robertson GW, Russell JD (December 1988). "5-Hydroxymaltol and mycophenolic acid, secondary metabolites from Penicillium echinulatum". Transactions of the British Mycological Society. 91 (4): 649–651. doi:10.1016/S0007-1536(88)80040-8.
- Fulton B, Markham A (February 1996). "Mycophenolate mofetil. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation". Drugs. 51 (2): 278–98. doi:10.2165/00003495-199651020-00007. PMID 8808168.
- Ransom JT (December 1995). "Mechanism of action of mycophenolate mofetil". Therapeutic Drug Monitoring. 17 (6): 681–4. doi:10.1097/00007691-199512000-00023. PMID 8588241.
- Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, et al. (November 2011). "Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis". The New England Journal of Medicine. 365 (20): 1886–95. doi:10.1056/NEJMoa1014460. PMID 22087680.
- W. Zhang, C. Ding, S. Enteric-coated mycophenolate sodium: an update Int J Clin Pract, April 2014, 68 (Suppl. 181), 1–3
- Lipsky JJ (November 1996). "Mycophenolate mofetil". Lancet. 348 (9038): 1357–9. doi:10.1016/S0140-6736(96)10310-X. PMID 8918281.
- Zhang L, Demain AL (2005). Natural Products: Drug Discovery and Therapeutic Medicine. Totowa, N.J.: Humana Press. p. 14. ISBN 978-1-59259-976-9.
- Regueira TB, Kildegaard KR, Hansen BG, Mortensen UH, Hertweck C, Nielsen J (May 2011). "Molecular basis for mycophenolic acid biosynthesis in Penicillium brevicompactum". Applied and Environmental Microbiology. 77 (9): 3035–43. doi:10.1128/AEM.03015-10. PMC 3126426. PMID 21398490.
- Bentley R (October 2000). "Mycophenolic Acid: a one hundred year odyssey from antibiotic to immunosuppressant". Chemical Reviews. 100 (10): 3801–26. doi:10.1021/cr990097b. PMID 11749328.
- Watts G (2014). "Anthony Clifford Allison". The Lancet. 383 (9925): 1290. doi:10.1016/S0140-6736(14)60635-8.
- Allison AC (May 2000). "Immunosuppressive drugs: the first 50 years and a glance forward". Immunopharmacology. 47 (2–3): 63–83. doi:10.1016/S0162-3109(00)00186-7. PMID 10878284.
- Allison AC, Kowalski WJ, Muller CD, Eugui EM (November 1993). "Mechanisms of action of mycophenolic acid". Annals of the New York Academy of Sciences. 696 (1): 63–87. Bibcode:1993NYASA.696...63A. doi:10.1111/j.1749-6632.1993.tb17143.x. PMID 7906496.
- Nelson PH, Eugui E, Wang CC, Allison AC (February 1990). "Synthesis and immunosuppressive activity of some side-chain variants of mycophenolic acid". Journal of Medicinal Chemistry. 33 (2): 833–8. doi:10.1021/jm00164a057. PMID 1967654.
- Eugui EM, Allison AC (June 1993). "Immunosuppressive activity of mycophenolate mofetil". Annals of the New York Academy of Sciences. 685 (1): 309–29. Bibcode:1993NYASA.685..309E. doi:10.1111/j.1749-6632.1993.tb35881.x. PMID 8363235.
- Allison AC, Eugui EM (February 1996). "Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF)". Clinical Transplantation. 10 (1 Pt 2): 77–84. PMID 8680053.
- Allison AC, Eugui EM (1993). "The design and development of an immunosuppressive drug, mycophenolate mofetil". Springer Seminars in Immunopathology. 14 (4): 353–80. doi:10.1007/bf00192309. PMID 8322167.
- Allison AC, Eugui EM (December 1993). "Immunosuppressive and other effects of mycophenolic acid and an ester prodrug, mycophenolate mofetil". Immunological Reviews. 136 (1): 5–28. doi:10.1111/j.1600-065x.1993.tb00652.x. PMID 7907572.
- Bechstein WO, Suzuki Y, Kawamura T, Jaffee B, Allison A, Hullett DA, Sollinger HW (1992). "Low-dose combination therapy of DUP-785 and RS-61443 prolongs cardiac allograft survival in rats". Transplant International. 5 Suppl 1 (Suppl 1): S482-3. doi:10.1111/tri.1992.5.s1.482. PMID 14621853.
- Kawamura T, Hullett DA, Suzuki Y, Bechstein WO, Allison AM, Sollinger HW (April 1993). "Enhancement of allograft survival by combination RS-61443 and DUP-785 therapy". Transplantation. 55 (4): 691–4, discussion 694–5. doi:10.1097/00007890-199304000-00001. PMID 8475537.
- Taylor DO, Ensley RD, Olsen SL, Dunn D, Renlund DG (1994). "Mycophenolate mofetil (RS-61443): preclinical, clinical, and three-year experience in heart transplantation". The Journal of Heart and Lung Transplantation. 13 (4): 571–82. PMID 7947873.
- "Risk Evaluation and Mitigation Strategy (REMS) Under Review for CellCept and Myfortic". U.S. Food and Drug Administration. Retrieved 23 July 2014.
- Donlon DM (15 June 1995). "New Agent to Prevent Kidney Transplant Rejection Now Available". Stanford University. Retrieved 23 July 2014.
- "CellCept registry data demonstrated superior long-term organ transplant outcomes". Roche.com. F. Hoffmann-La Roche Ltd. Archived from the original on 26 July 2014. Retrieved 23 July 2014.
- "CellCept EPAR". European Medicines Agency (EMA). Retrieved 6 April 2020.
- Mimouni D, Anhalt GJ, Cummins DL, Kouba DJ, Thorne JE, Nousari HC (June 2003). "Treatment of pemphigus vulgaris and pemphigus foliaceus with mycophenolate mofetil". Archives of Dermatology. 139 (6): 739–42. doi:10.1001/archderm.139.6.739. PMID 12810504.
- Diamond MS, Zachariah M, Harris E (December 2002). "Mycophenolic acid inhibits dengue virus infection by preventing replication of viral RNA". Virology. 304 (2): 211–21. doi:10.1006/viro.2002.1685. PMID 12504563.
- Takhampunya R, Ubol S, Houng HS, Cameron CE, Padmanabhan R (July 2006). "Inhibition of dengue virus replication by mycophenolic acid and ribavirin". The Journal of General Virology. 87 (Pt 7): 1947–52. doi:10.1099/vir.0.81655-0. PMID 16760396.
- Chan JF, Chan KH, Kao RY, To KK, Zheng BJ, Li CP, et al. (December 2013). "Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus". The Journal of Infection. 67 (6): 606–16. doi:10.1016/j.jinf.2013.09.029. PMID 24096239.
- Xiao, Yousheng; Huang, Jianyi; Luo, Hongye; Wang, Jin (2014-02-07). "Mycophenolate mofetil for relapsing-remitting multiple sclerosis". The Cochrane Database of Systematic Reviews (2): CD010242. doi:10.1002/14651858.CD010242.pub2. ISSN 1469-493X. PMID 24505016.
External links
- "Mycophenolic acid". Drug Information Portal. U.S. National Library of Medicine.
- "Mycophenolate mofetil". Drug Information Portal. U.S. National Library of Medicine.
- "Mycophenolate sodium". Drug Information Portal. U.S. National Library of Medicine.