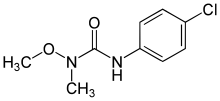
Monolinuron
Monolinuron is a pesticide,[1] more specifically a selective systemic herbicide[2] and an algaecide.[3] As an herbicide, it is used to control broad-leaved weeds and annual grasses in vegetable crops such as leeks, potatoes,[2] and dwarf French beans.[4] Monolinuron affects the photosynthesis in weeds. Following uptake of monolinuron through roots and leaves of weeds, monolinuron causes early symptoms of yellowing and die-back of the leaves, eventually resulting in weed death.[4] In fishkeeping it is used to control blanketweed and hair algae.[3]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-(4-Chlorophenyl)-1-methoxy-1-methylurea | |
| Systematic IUPAC name
1-(4-Chlorophenyl)-3-methoxy-3-methylurea | |
| Other names
N′-(4-Chlorophenyl)-N-methoxy-N-methylurea | |
| Identifiers | |
3D model (JSmol) |
|
| 2212523 | |
| ChemSpider | |
| ECHA InfoCard | 100.015.572 |
| EC Number |
|
| KEGG | |
| MeSH | Monolinuron |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H11ClN2O2 | |
| Molar mass | 214.65 g·mol−1 |
| Melting point | 80 to 83 °C (176 to 181 °F; 353 to 356 K) |
| 0.735 g/L | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
As a herbicide it is used in a rage of food and non-food crops, e.g. beans and other vegetables, onions, fruits, cereals, potatoes, vines and ornamental plants. It was introduced to use in circa 1965. It is an inhibitor of photosystem II of photosynthesis. It is a derivative of urea and related to paraquat. It is available under trae names e.g. Monamex, Gramonol, Aresin.[5]
It is soluble in water (0.735 g/l), and very soluble in organic solvents (200 g/l in acetone, methanol, and toluene, 3.9 g/l in hexane). Its volatility is low and its leachability is high. It is moderately persistent and moderately mobile in soil, stable in water, and fast degraded in water sediments (DT50 of 22 days). It has low toxicity for mammals (LD50 oral for a rat is 2100 mg/kg).[5]
References
- Rossoff; Irving S. (2002). Encyclopedia of clinical toxicology. p. 718.
- Milne; George W. A. (2005). Gardner's commercially important chemicals. p. 44.
- "Pesticides: HSE registered products". Archived from the original on 2009-07-24. Retrieved 2009-07-31.
- "Department for Environment, Food and Rural Affairs, Evaluation of Fully Approved or Provisionally Approved Products, Evaluation on: Monolinuron, May 1995" (PDF). Retrieved 2011-08-19.
- https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/475.htm