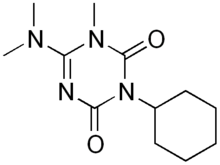
Hexazinone
Hexazinone is an organic compound that is used as a broad spectrum herbicide. It is a colorless solid. It exhibits some solubility in water but is highly soluble in most organic solvents except alkanes. A member of the triazine class herbicides, it is manufactured by DuPont and sold under the trade name Velpar.[1]
 | |
| Names | |
|---|---|
| IUPAC name
3-Cyclohexyl-6-dimethylamino-1-methyl-1,3,5-triazine-2,4-dione | |
| Other names
Velpar Hexazinone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.051.869 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H20N4O2 | |
| Molar mass | 252.31 |
| Appearance | White crystalline solid |
| Density | 1.25 g/cm3 |
| Melting point | 116 °C (241 °F; 389 K) |
| Soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It functions by inhibiting photosynthesis and thus is a nonselective herbicide. It is used to control grasses, broadleaf, and woody plants. Approximately 33% is used on alfalfa, 31% in forestry, 29% in industrial areas, 4% on rangeland and pastures, and < 2% on sugarcane.[2]
Hexazinone is a pervasive groundwater contaminant, due to its high water solubility
History
Hexazinone is widely used as a herbicide. It is a non-selective herbicide from the triazine family. It is used among a broad range of places. It is used to control weeds within all sort of applications. From sugarcane plantations, forestry field nurseries, pineapple plantations to high- and railway grasses and industrial plant sites.[3]
Hexazinone was first registered in 1975 for the overall control of weeds and later for uses in crops.[4]
Structure and reactivity
Triazines like hexazinone can bind to the D-1 quinone protein of the electron transport chain in photosystem II to inhibit the photosynthesis. These diverted electrons can thereby damage membranes and destroy cells.[5]
Synthesis
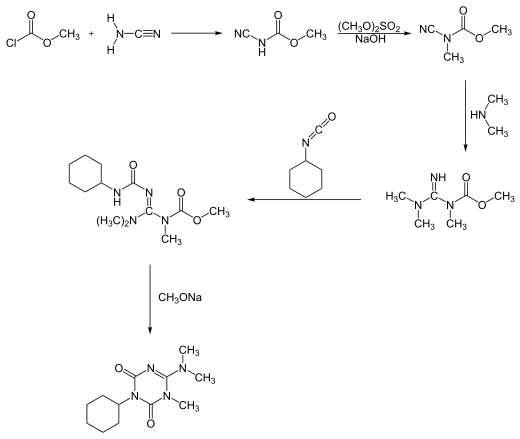
Hexazinone can be synthesized in two different reaction processes. One process starts with a reaction of methyl chloroformate with cyanamide, forming hexazinone after a five-step pathway:[6]
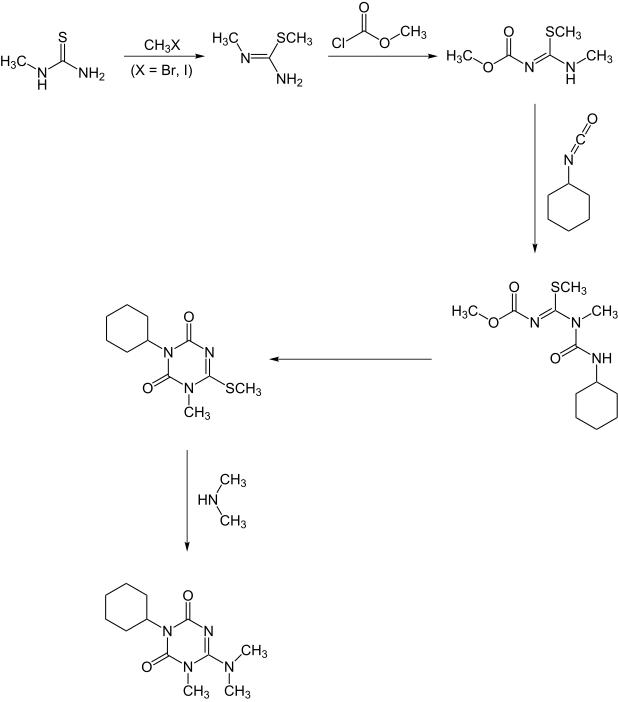
A second synthesis starts with methylthiourea.:[6]
Degradation
The degradation of hexazinone has long been studied.[7] It degrades approximately 10% in five weeks, when exposed to artificial sunlight in distilled water. However, degradation in natural waters can be three to seven times greater. Surprisingly, the pH and the temperature of the water do not affect the photodegradation significantly.[8] It is mainly degraded by aerobic microorganisms in soils.[9]
Mechanism of action
Hexazinone is a broad-spectrum residual and contact herbicide, rapidly absorbed by the leaves and roots. It is tolerated by conifers, and therefore it is a very effective herbicide for the control for annual and perennial broadleaf weeds, some grasses, and some woody species. Hexazinone works as rain or snowmelt makes it possible for the herbicide to move downward into the soil. There the hexazinone is absorbed from the soil by the roots.[10] It moves through the conductive tissues to the leaves, where it blocks the photosynthesis of the plant within the chloroplasts. Hexazinone binds to a protein of the photosystem II complex, which blocks the electron transport. The result are multiple following reactions. First triplet-state chlorophyll reacts with oxygen to form singlet oxygen. Both chlorophyll and singlet oxygen then remove hydrogen ions from the unsaturated lipids present in de cells and the organelle membranes, forming lipid radicals. These radicals will oxidize other lipids and proteins, eventually resulting in loss of the membrane integrity of the cells and organelles. This will result in a loss of chlorophyll, leakage of cellular contents, cell death, and eventually death of the plant.[11] Woody plants first show yellowing of the leaves before they start to defoliate, eventually they will die.[12] Sometimes plants are able to refoliate and defoliate again during the growing season.
References
- Arnold P. Appleby, Franz Müller, Serge Carpy "Weed Control" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a28_165
- Hexazinone, Herbicide Profile, Pesticide Management Education Program, Cornell University
- Wang, Huili; Xu, Shuxia; Tan, Chengxia; Wang, Xuedong (2009-05-30). "Anaerobic biodegradation of hexazinone in four sediments". Journal of Hazardous Materials. 164 (2–3): 806–811. doi:10.1016/j.jhazmat.2008.08.073. PMID 18824297.
- "Hexazinone: Reregistration Eligibility Decision (RED) Fact Sheet" (PDF).
- "Agronomy 317 - Iowa State University". agron-www.agron.iastate.edu. Archived from the original on 2016-11-23. Retrieved 2017-03-15.
- Ullmann's agrochemicals. Wiley-VCH. 2007-01-01. ISBN 9783527316045. OCLC 470787466.
- Helling, C. S.; Kearney, P. C.; Alexander, M. (1971). "Behavior of pesticides in soil". Adv. Agron. Advances in Agronomy. 23: 147–240. doi:10.1016/S0065-2113(08)60153-4. ISBN 9780120007233.
- Rhodes, R. C. (1980b). "Studies with 14C-labeled hexazinone in water and bluegill sunfish". J. Agric. Food Chem. 28 (2): 306–310. doi:10.1021/jf60228a002. PMID 7391368.
- Rhodes, R. C. (1980a). "Soil Studies with 14C-labeled hexazinone". J. Agric. Food Chem. 28 (2): 311–315. doi:10.1021/jf60228a012.
- Ghassemi, M.; et al. (1981). Environmental fates and impacts of major forest use pesticides. Washington D.C. pp. 169–194.
- "Weed Science Society of America". wssa.net. Retrieved 2017-03-15.
- Sidhu, S. S.; Feng., J. C. (1993). "Hexazinone and its metabolites in boreal forest vegetation". Weed Sci. 41 (2): 281–287. doi:10.1017/S0043174500076177.
External links
- DuPont webpage on Velpar
- Hexazinone in the Pesticide Properties DataBase (PPDB)