Methylidene group
In organic chemistry, a methylidene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon atom, which is connected to the remainder of the molecule by a double bond.[1] [2] The group may be represented as CH2=, where the '=' denotes the double bond.
This stands in contrast to methylene, also called methylene bridge, i.e. the −CH2− group, which is connected to the rest of the molecule by two single bonds.[3][4] The distinction is often important, because the double bond is chemically different from two single bonds.
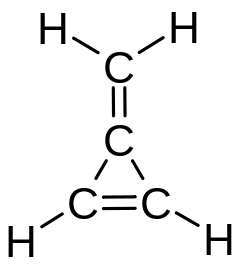
The same name (methylidene) is used for the radical CH
2, which is a molecule unto itself, also known as carbene.[5][6] Formerly the methylene name was used for all three isomers (methylene, methylidene, and carbene).
Many organic compounds are named and classified as if they were the result of substituting a methylidene group for two adjacent hydrogen atoms of some parent molecule (even if they are not actually obtained that way). Thus, for example, methylenecyclopropene is named after cyclopropene.
References