Mechanoreceptor
A mechanoreceptor, also called mechanoceptor, is a sensory cell that responds to mechanical pressure or distortion. There are four main types of mechanoreceptors in glabrous, or hairless, mammalian skin: lamellar corpuscles (Pacinian corpuscles), tactile corpuscles (Meissner's corpuscles), Merkel nerve endings, and bulbous corpuscles (Ruffini corpuscle). There are also mechanoreceptors in hairy skin, and the hair cells in the receptors of primates like rhesus monkeys and other mammals are similar to those of humans and also studied even in early 20th century anatomically and neurophysiologically.[1]
Invertebrate mechanoreceptors include campaniform sensilla and slit sensilla, among others.
Mechanoreceptors are also present in plant cells where they play an important role in normal growth, development and the sensing of their environment.[2]
Mechanism of sensation
In somatosensory transduction, the afferent neurons transmit messages through synapses in the dorsal column nuclei, where second-order neurons send the signal to the thalamus and synapse with third-order neurons in the ventrobasal complex. The third-order neurons then send the signal to the somatosensory cortex.
Feedback
More recent work has expanded the role of the cutaneous mechanoreceptors for feedback in fine motor control.[3] Single action potentials from Meissner's corpuscle, Pacinian corpuscle and Ruffini ending afferents are directly linked to muscle activation, whereas Merkel cell-neurite complex activation does not trigger muscle activity.[4]
Types
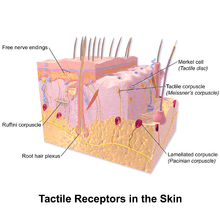
In glabrous (hairless) skin, there are four principal types of mechanoreceptors, each shaped according to its function. The tactile corpuscles (also known as Meissner corpuscles) respond to light touch, and adapt rapidly to changes in texture (vibrations around 50 Hz). The bulbous corpuscles (also known as Ruffini endings) detect tension deep in the skin and fascia. The Merkel nerve endings (also known as Merkel discs) detect sustained pressure. The lamellar corpuscles (also known as Pacinian corpuscles) in the skin and fascia detect rapid vibrations (of about 200–300 Hz).
Receptors in hair follicles called hair root plexuses[5] sense when a hair changes position. Indeed, the most sensitive mechanoreceptors in humans are the hair cells in the cochlea of the inner ear (no relation to the follicular receptors – they are named for the hair-like mechanosensory stereocilia they possess); these receptors transduce sound for the brain.
Mechanosensory free nerve endings detect touch, pressure, stretching, as well as the tickle and itch sensations. Itch sensations are caused by stimulation of free nerve ending from chemicals.[5]
Baroreceptors are a type of mechanoreceptor sensory neuron that is excited by stretch of the blood vessel.
Cutaneous
Cutaneous mechanoreceptors respond to mechanical stimuli that result from physical interaction, including pressure and vibration. They are located in the skin, like other cutaneous receptors. They are all innervated by Aβ fibers, except the mechanorecepting free nerve endings, which are innervated by Aδ fibers. Cutaneous mechanoreceptors can be categorized by morphology, by what kind of sensation they perceive, and by the rate of adaptation. Furthermore, each has a different receptive field.
- The Slowly Adapting type 1 (SA1) mechanoreceptor, with the Merkel corpuscle end-organ, underlies the perception of form and roughness on the skin.[6] They have small receptive fields and produce sustained responses to static stimulation.
- The Slowly Adapting type 2 (SA2) mechanoreceptors, with the Ruffini corpuscle end-organ, respond to skin stretch, but have not been closely linked to either proprioceptive or mechanoreceptive roles in perception.[7] They also produce sustained responses to static stimulation, but have large receptive fields.
- The Rapidly Adapting (RA) or Meissner corpuscle end-organ mechanoreceptor underlies the perception of flutter[8] and slip on the skin.[9] They have small receptive fields and produce transient responses to the onset and offset of stimulation.
- The Pacinian corpuscle or Vater-Pacinian corpuscles or Lamellar corpuscles[10] underlie the perception of high frequency vibration.[8][11] They also produce transient responses, but have large receptive fields.
By sensation
By rate of adaptation
Cutaneous mechanoreceptors can also be separated into categories based on their rates of adaptation. When a mechanoreceptor receives a stimulus, it begins to fire impulses or action potentials at an elevated frequency (the stronger the stimulus, the higher the frequency). The cell, however, will soon "adapt" to a constant or static stimulus, and the pulses will subside to a normal rate. Receptors that adapt quickly (i.e. quickly return to a normal pulse rate) are referred to as "phasic". Those receptors that are slow to return to their normal firing rate are called tonic. Phasic mechanoreceptors are useful in sensing such things as texture or vibrations, whereas tonic receptors are useful for temperature and proprioception among others.
- Slowly adapting: Slowly adapting mechanoreceptors include Merkel and Ruffini corpuscle end-organs, and some free nerve endings.
- Slowly adapting type I mechanoreceptors have multiple Merkel corpuscle end-organs.
- Slowly adapting type II mechanoreceptors have single Ruffini corpuscle end-organs.
- Intermediate adapting: Some free nerve endings are intermediate adapting.
- Rapidly adapting: Rapidly adapting mechanoreceptors include Meissner corpuscle end-organs, Pacinian corpuscle end-organs, hair follicle receptors and some free nerve endings.
- Rapidly adapting type I mechanoreceptors have multiple Meissner corpuscle end-organs.
- Rapidly adapting type II mechanoreceptors (usually called Pacinian) have single Pacinian corpuscle end-organs.
Receptive field
Cutaneous mechanoreceptors with small, accurate receptive fields are found in areas needing accurate taction (e.g. the fingertips). In the fingertips and lips, innervation density of slowly adapting type I and rapidly adapting type I mechanoreceptors are greatly increased. These two types of mechanoreceptors have small discrete receptive fields and are thought to underlie most low-threshold use of the fingers in assessing texture, surface slip, and flutter. Mechanoreceptors found in areas of the body with less tactile acuity tend to have larger receptive fields.
Others
Other mechanoreceptors than cutaneous ones include the hair cells, which are sensory receptors in the vestibular system of the inner ear, where they contribute to the auditory system and equilibrioception. In addition to this, mechanoreceptors aid Dionaea muscipula Ellis (Venus Fly Trap) in capturing sizable[12] prey effectively.[13]
There are also Juxtacapillary (J) receptors, which respond to events such as pulmonary edema, pulmonary emboli, pneumonia, and barotrauma.
Ligamentous
There are four types of mechanoreceptors embedded in ligaments. As all these types of mechanoreceptors are myelinated, they can rapidly transmit sensory information regarding joint positions to the central nervous system.[14]
- Type I: (small) Low threshold, slow adapting in both static and dynamic settings
- Type II: (medium) Low threshold, rapidly adapting in dynamic settings
- Type III: (large) High threshold, slowly adapting in dynamic settings
- Type IV: (very small) High threshold pain receptors that communicate injury
Type II and Type III mechanoreceptors in particular are believed to be linked to one's sense of proprioception.
Lamellar corpuscle
Lamellar corpuscles, or Pacinian corpuscles, are pressure receptors located in the skin and also in various internal organs. Each is connected to a sensory neuron. Because of its relatively large size, a single lamellar corpuscle can be isolated and its properties studied. Mechanical pressure of varying strength and frequency can be applied to the corpuscle by stylus, and the resulting electrical activity detected by electrodes attached to the preparation.
Deforming the corpuscle creates a generator potential in the sensory neuron arising within it. This is a graded response: the greater the deformation, the greater the generator potential. If the generator potential reaches threshold, a volley of action potentials (nerve impulses) are triggered at the first node of Ranvier of the sensory neuron.
Once threshold is reached, the magnitude of the stimulus is encoded in the frequency of impulses generated in the neuron. So the more massive or rapid the deformation of a single corpuscle, the higher the frequency of nerve impulses generated in its neuron.
The optimal sensitivity of a lamellar corpuscle is 250 Hz, the frequency range generated upon finger tips by textures made of features smaller than 200 micrometres.[15]
Muscle spindles and the stretch reflex
The knee jerk is the popularly known stretch reflex (involuntary kick of the lower leg) induced by tapping the knee with a rubber-headed hammer. The hammer strikes a tendon that inserts an extensor muscle in the front of the thigh into the lower leg. Tapping the tendon stretches the thigh muscle, which activates stretch receptors within the muscle called muscle spindles. Each muscle spindle consists of sensory nerve endings wrapped around special muscle fibers called spindle fibers (also called intrafusal fibers). Stretching a spindle fiber initiates a volley of impulses in the sensory neuron (a I-a neuron) attached to it. The impulses travel along the sensory axon to the spinal cord where they form several kinds of synapses:
- Some of the branches of the I-a axons synapse directly with alpha motor neurons.These carry impulses back to the same muscle causing it to contract. The leg straightens.
- Some of the branches of the I-a axons synapse with inhibitory interneurons in the spinal cord. These, in turn, synapse with motor neurons leading back to the antagonistic muscle, a flexor in the back of the thigh. By inhibiting the flexor, these interneurons aid contraction of the extensor.
- Still other branches of the I-a axons synapse with interneurons leading to brain centers, e.g., the cerebellum, that coordinate body movements.[16]
See also
Notes
- Adrian ED, Umrath K (October 1929). "The impulse discharge from the pacinian corpuscle". The Journal of Physiology. 68 (2): 139–54. doi:10.1113/jphysiol.1929.sp002601. PMC 1402853. PMID 16994055.
- Monshausen, Gabriele B.; Haswell, Elizabeth S. (December 2013). "A force of nature: molecular mechanisms of mechanoperception in plants". Journal of Experimental Botany. 64 (15): 4663–4680. doi:10.1093/jxb/ert204. ISSN 0022-0957. PMC 3817949. PMID 23913953.
- Johansson RS, Flanagan JR (May 2009). "Coding and use of tactile signals from the fingertips in object manipulation tasks" (PDF). Nature Reviews. Neuroscience. 10 (5): 345–59. doi:10.1038/nrn2621. PMID 19352402.
- McNulty PA, Macefield VG (December 2001). "Modulation of ongoing EMG by different classes of low-threshold mechanoreceptors in the human hand". The Journal of Physiology. 537 (Pt 3): 1021–32. doi:10.1111/j.1469-7793.2001.01021.x. PMC 2278990. PMID 11744774.
- Tortora, Gerard J., author. Principles of anatomy and physiology. ISBN 978-0-7303-5500-7. OCLC 1059417106.CS1 maint: multiple names: authors list (link)
- Johnson KO, Hsiao SS (1992). "Neural mechanisms of tactual form and texture perception". Annual Review of Neuroscience. 15: 227–50. doi:10.1146/annurev.ne.15.030192.001303. PMID 1575442.
- Torebjörk HE, Ochoa JL (December 1980). "Specific sensations evoked by activity in single identified sensory units in man". Acta Physiologica Scandinavica. 110 (4): 445–7. doi:10.1111/j.1748-1716.1980.tb06695.x. PMID 7234450.
- Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB (March 1968). "The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand". Journal of Neurophysiology. 31 (2): 301–34. doi:10.1152/jn.1968.31.2.301. PMID 4972033.
- Johansson RS, Westling G (1987). "Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip". Experimental Brain Research. 66 (1): 141–54. doi:10.1007/bf00236210. PMID 3582528.
- Biswas A, Manivannan M, Srinivasan MA (2015). "Multiscale layered biomechanical model of the pacinian corpuscle". IEEE Transactions on Haptics. 8 (1): 31–42. doi:10.1109/TOH.2014.2369416. PMID 25398182.
- Biswas A, Manivannan M, Srinivasan MA (2015). "Vibrotactile sensitivity threshold: nonlinear stochastic mechanotransduction model of the Pacinian Corpuscle". IEEE Transactions on Haptics. 8 (1): 102–13. doi:10.1109/TOH.2014.2369422. PMID 25398183.
- Chamovitz D (2012). What a plant knows : a field guide to the senses (1st ed.). New York: Scientific American/Farrar, Straus and Giroux. ISBN 9780374533885. OCLC 755641050.
- Volkov AG, Forde-Tuckett V, Volkova MI, Markin VS (2014-02-10). "Morphing structures of the Dionaea muscipula Ellis during the trap opening and closing". Plant Signaling & Behavior. 9 (2): e27793. doi:10.4161/psb.27793. PMC 4091236. PMID 24618927.
- Michelson JD, Hutchins C (March 1995). "Mechanoreceptors in human ankle ligaments". The Journal of Bone and Joint Surgery. British Volume. 77 (2): 219–24. doi:10.1302/0301-620X.77B2.7706334. PMID 7706334.
- Scheibert J, Leurent S, Prevost A, Debrégeas G (March 2009). "The role of fingerprints in the coding of tactile information probed with a biomimetic sensor". Science. 323 (5920): 1503–6. arXiv:0911.4885. Bibcode:2009Sci...323.1503S. doi:10.1126/science.1166467. PMID 19179493.
- Kimball JW (2011). "Mechanoreceptors". Kimball's Biology Pages. Archived from the original on 27 February 2011.
External links
| Look up mechanoreceptor in Wiktionary, the free dictionary. |
- Mechanoreceptors at the US National Library of Medicine Medical Subject Headings (MeSH)