Isotopes of magnesium
Magnesium (12Mg) naturally occurs in three stable isotopes, 24Mg, 25Mg, and 26Mg. There are 18 radioisotopes that have been discovered, ranging from 19Mg to 40Mg. The longest-lived radioisotope is 28Mg with a half-life of 20.915 hours. The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. The shortest-lived is 19Mg with a half-life of 5 picoseconds.
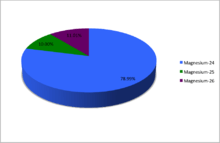
The abundances of the naturally occurring isotopes of magnesium.
| ||||||||||||||||||||||||||||||
| Standard atomic weight Ar, standard(Mg) |
| |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
List of isotopes
| Nuclide[2] |
Z | N | Isotopic mass (Da)[3] [n 1] |
Half-life [n 2] |
Decay mode [n 3] |
Daughter isotope [n 4] |
Spin and parity [n 5][n 2] |
Natural abundance (mole fraction) | |
|---|---|---|---|---|---|---|---|---|---|
| Normal proportion | Range of variation | ||||||||
| 19Mg | 12 | 7 | 19.03417(5) | 5(3) ps | 2p | 17Ne | 1/2−# | ||
| 20Mg | 12 | 8 | 20.0187631(2) | 93(5) ms | β+ (69.7%) | 20Na | 0+ | ||
| β+, p (30.3%) | 19Ne | ||||||||
| 21Mg | 12 | 9 | 21.0117058(8) | 118.6(5) ms | β+ (66.9%) | 21Na | 5/2+ | ||
| β+, p (32.6%) | 20Ne | ||||||||
| β+, α (0.5%) | 17F | ||||||||
| 22Mg | 12 | 10 | 21.9995707(3) | 3.8755(12) s | β+ | 22Na | 0+ | ||
| 23Mg | 12 | 11 | 22.99412394(17) | 11.317(11) s | β+ | 23Na | 3/2+ | ||
| 24Mg | 12 | 12 | 23.985041697(14) | Stable | 0+ | 0.7899(4) | 0.78958–0.79017 | ||
| 25Mg | 12 | 13 | 24.98583696(5) | Stable | 5/2+ | 0.1000(1) | 0.09996–0.10012 | ||
| 26Mg[n 6] | 12 | 14 | 25.98259297(3) | Stable | 0+ | 0.1101(3) | 0.10987–0.11030 | ||
| 27Mg | 12 | 15 | 26.98434063(5) | 9.435(27) min | β− | 27Al | 1/2+ | ||
| 28Mg | 12 | 16 | 27.9838766(21) | 20.915(9) h | β− | 28Al | 0+ | ||
| 29Mg | 12 | 17 | 28.988617(12) | 1.30(12) s | β− | 29Al | 3/2+ | ||
| 30Mg | 12 | 18 | 29.990463(4) | 313(4) ms | β− (99.94%) | 30Al | 0+ | ||
| β−, n (0.06%) | 29Al | ||||||||
| 31Mg | 12 | 19 | 30.996648(3) | 236(20) ms | β− (93.8%) | 31Al | 1/2(+) | ||
| β−, n (6.2%) | 30Al | ||||||||
| 32Mg | 12 | 20 | 31.999110(4) | 86(5) ms | β− (94.5%) | 32Al | 0+ | ||
| β−, n (5.5%) | 31Al | ||||||||
| 33Mg | 12 | 21 | 33.005327(3) | 90.5(16) ms | β− (86%) | 33Al | 3/2− | ||
| β−, n (14%) | 32Al | ||||||||
| 34Mg | 12 | 22 | 34.00894(3) | 20(10) ms | β− (70%) | 34Al | 0+ | ||
| β−, n (30%) | 33Al | ||||||||
| 35Mg | 12 | 23 | 35.01679(29) | 70(40) ms | β−, n (52%) | 34Al | 7/2−# | ||
| β− (48%) | 35Al | ||||||||
| 36Mg | 12 | 24 | 36.02188(74) | 3.9(13) ms | β− | 36Al | 0+ | ||
| 37Mg | 12 | 25 | 37.03029(75) | 8(4) ms | β− | 37Al | (3/2−) | ||
| β−, n | 36Al | ||||||||
| 38Mg | 12 | 26 | 38.03658(54)# | 1# ms [>260 ns] | 0+ | ||||
| 40Mg | 12 | 28 | 40.05191(54)# | 1# ms | β−, n | 39Al | 0+ | ||
| β− | 40Al | ||||||||
- ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding last digits.
- # – Values marked # are not purely derived from experimental data, but at least partly from trends of neighboring nuclides (TNN).
-
Modes of decay:
n: Neutron emission p: Proton emission - Bold symbol as daughter – Daughter product is stable.
- ( ) spin value – Indicates spin with weak assignment arguments.
- Used in radiodating events early in the Solar System's history
gollark: It can be called 5.
gollark: Idea: a language with the operation `5`, which prints 5.
gollark: ^
gollark: So will I.
gollark: Too bad.
References
- Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- Half-life, decay mode, nuclear spin, and isotopic composition is sourced in:
Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001. - Wang, M.; Audi, G.; Kondev, F. G.; Huang, W. J.; Naimi, S.; Xu, X. (2017). "The AME2016 atomic mass evaluation (II). Tables, graphs, and references" (PDF). Chinese Physics C. 41 (3): 030003-1–030003-442. doi:10.1088/1674-1137/41/3/030003.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.