Isomer
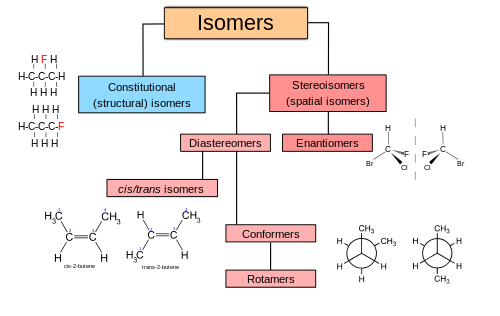
In chemistry, isomers are molecules or polyatomic ions with identical chemical formulas but distinct arrangements of atoms in space.[1] Isomers do not necessarily share similar properties. Two main forms of isomerism are structural isomerism (or constitutional isomerism, in which bonds differ) and stereoisomerism (or spatial isomerism, in which the orientations of atoms differ).
Structural isomers
Structural isomers differ in terms of the connectivity of some or all constituent atoms.[2]
Example: propanols and methoxyethane
A simple example of isomerism is given by propanol: It has the formula C3H8O (or C3H7OH) and occurs as two isomers: propan-1-ol (n-propyl alcohol; I) and propan-2-ol (isopropyl alcohol; II)
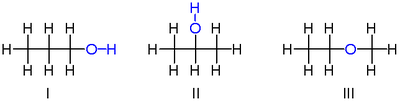
These two molecules are position isomers of each other, because the position of the hydroxy group differs between the two: It is attached to an end carbon in the first isomer, and to the center carbon in the second.
Another isomer of C3H8O: methoxyethane (ethyl-methyl-ether; III). Unlike the isomers of propanol, methoxyethane has an oxygen connected to two carbons rather than to one carbon and one hydrogen. Methoxyethane is an ether, not an alcohol, because it lacks a hydroxyl group, and it has chemical properties more similar to other ethers than to either of the above alcohol isomers.
Example: propadiene and propyne
Propadiene (or allene) and propyne (or methylacetylene) are examples of isomers containing different bond types. Propadiene contains two double bonds, whereas propyne contains one triple bond.
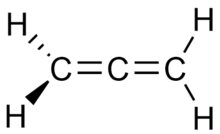 Propadiene |
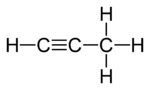 Propyne |
Tautomers
Tautomers are structural isomers which readily interconvert, so that two or more species co-exist in equilibria such as H–X–Y=Z ⇌ X=Y–Z–H.[3] Important examples are keto-enol tautomerism and the equilibrium between neutral and zwitterionic forms of an amino acid.
Stereoisomers
Stereoisomers have the same bond connectivity but distinct geometries. Two broad classes of stereoisomers are recognized: enantiomers and diastereomers. Enantiomers are non-superposable mirror-images of each other. Diastereomers are not. Enantiomers always contain chiral centers. Some diastereomers are chiral and some are not.[4] Another type of isomer, conformational isomers (conformers), may be rotamers, diastereomers, or enantiomers depending on the compound. For example, ortho- position-locked biphenyl systems have enantiomers.
E/Z isomers, which have restricted rotation at a double bond, are configurational isomers. They are classified as diastereomers.[4] E/Z notation depicts absolute stereochemistry, which is an unambiguous descriptor based on CIP priorities.
"Cis–trans isomers" are used to describe any molecules with restricted rotation in the molecule. For alkenes, these descriptors describe relative stereochemistry so can be ambiguous. This terminology is especially problematic for double bonds that have more than two substituents. An obsolete term for cis–trans isomerism is "geometric isomerism".[5] For alkenes with more than two substituents, E-Z notation is used instead of cis and trans. If possible, E and Z (written in italic type) is also preferred in compounds with two substituents.[6]
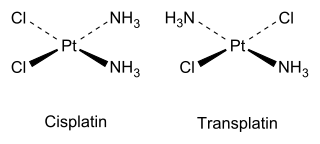
Cis and trans isomers also occur in inorganic coordination compounds, such as square planar MX2Y2 complexes and octahedral MX4Y2 complexes. A related type of geometric isomerism is facial–meridional (fac-mer) isomerism in octahedral MX3Y3 complexes, in which each set of three identical ligands either occupies one face of the octahedron or is situated on one meridian of the octahedron.
Although conformers can be referred to as stereoisomers, they are rarely isolable in pure form.
While structural isomers typically have distinct chemical properties, stereoisomers behave identically in most chemical reactions, except in their reaction with other stereoisomers. Enzymes, however, can distinguish between enantiomers of a compound.
Enantiomers differ in the sign of their optical rotation of polarized light, and are therefore sometimes described as optical isomers.[7][8] However this term is ill-defined and has also been used to describe other stereoisomers as well as enantiomers.[9] Its use is therefore strongly discouraged and the molecules should instead be described as either enantiomers or diastereomers as appropriate.[10][9]
Isomerization
Isomerization is the process by which one molecule is transformed into another molecule that has exactly the same atoms, but the atoms are rearranged.[11] In some molecules and under some conditions, isomerization occurs spontaneously. Many isomers are equal or roughly equal in bond energy, and so exist in roughly equal amounts, provided that they can interconvert relatively freely, that is the energy barrier between the two isomers is not too high. When the isomerization occurs intramolecularly, it is considered a rearrangement reaction.
An example of an organometallic isomerization is the production of decaphenylferrocene, [(η5-C5Ph5)2Fe] from its linkage isomer.[12][13]
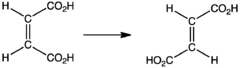
- Synthesis of fumaric acid
Industrial synthesis of fumaric acid proceeds via the cis-trans isomerization of maleic acid:
Medicinal chemistry
Isomers having distinct biological properties are common; for example, the placement of methyl groups. In substituted xanthines, theobromine, found in chocolate, is a vasodilator with some effects in common with caffeine; but, if one of the two methyl groups is moved to a different position on the two-ring core, the isomer is theophylline, which has a variety of effects, including bronchodilation and anti-inflammatory action. Another example of this occurs in the phenethylamine-based stimulant drugs. Phentermine is a non-chiral compound with a weaker effect than that of amphetamine. It is used as an appetite-reducing medication and has mild or no stimulant properties. However, an alternate atomic arrangement gives dextromethamphetamine, which is a stronger stimulant than amphetamine.
In medicinal chemistry and biochemistry, enantiomers are a special concern because they may possess distinct biological activity. Many preparative procedures afford a mixture of equal amounts of both enantiomeric forms. In some cases, the enantiomers are separated by chromatography using chiral stationary phases. They may also be separated through the formation of diastereomeric salts. In other cases, enantioselective synthesis have been developed.
As an inorganic example, cisplatin (see structure above) is an important drug used in cancer chemotherapy, whereas the trans isomer (transplatin) has no useful pharmacological activity.
History
Isomerism was first observed in 1827, when Friedrich Wöhler prepared silver cyanate and discovered that, although its elemental composition of AgCNO was identical to silver fulminate (prepared by Justus von Liebig the previous year),[14] its properties were distinct. This finding challenged the prevailing chemical understanding of the time, which held that chemical compounds could be distinct only when their elemental compositions differ. After additional discoveries of the same sort were made, such as Wöhler's 1828 discovery that urea has the same atomic composition (CH4N2O) as the chemically distinct ammonium cyanate, Jöns Jacob Berzelius introduced the term isomerism in 1830 to describe the phenomenon.[15] The isomeric pairs are as follows:
Chemical Structure Chemical Structure Silver fulminate Ammonium cyanate Silver cyanate Urea
In 1848, Louis Pasteur separated tartaric acid into tiny crystals of its two mirror-image forms.[16][17] The individual molecules of each were the left and right optical stereoisomers, solutions of which rotate the plane of polarized light to the same degree but in opposite directions.
Other types of isomerism
Other types of isomerism exist. In general, topological isomers called topoisomers are large molecules that wind about and form knots or loops. Molecules with topoisomers include catenanes and DNA. Topoisomerase enzymes can knot DNA and thus change its topology. There are also isotopomers or isotopic isomers that have the same numbers of each type of isotopic substitution but in chemically different positions. In nuclear physics, nuclear isomers are excited states of atomic nuclei. Spin isomers have differing distributions of spin among their constituent atoms.
Etymology
Isomer (/ˈaɪsəmər/; from Greek ἰσομερής, isomerès; isos = "equal", méros = "part") is the root of "isomer". The term was coined by Swedish chemist Jöns Jacob Berzelius (1779–1848) in 1830. [18] Isomerism (/ˈaɪsəməˌrɪzəm, aɪˈsɒ-/) refers to the existence of isomers.
See also
- Chirality (chemistry)
- Cis-trans isomerism
- Cyclohexane conformation
- Electromerism
- Isomery (botany)
- Ligand isomerism
- Nuclear isomer
- Stereocenter
- Structural isomerism
- Tautomer
- Vitamer
References
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General chemistry: principles and modern applications (8th ed.). Upper Saddle River, N.J: Prentice Hall. p. 91. ISBN 978-0-13-014329-7. LCCN 2001032331. OCLC 46872308.CS1 maint: ref=harv (link)
- Smith, Janice Gorzynski (2010). General, Organic and Biological Chemistry (1st ed.). McGraw-Hill. p. 450. ISBN 978-0-07-302657-2.
- "tautomerism". IUPAC Gold Book. IUPAC. Retrieved 21 April 2019.
- Ernest L. Eliel and Samuel H. Wilen (1994). Stereochemistry of Organic Compounds. Wiley Interscience. pp. 52–53.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "geometric isomerism". doi:10.1351/goldbook.G02620
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "cis, trans". doi:10.1351/goldbook.C01092
- Petrucci, Harwood & Herring 2002, pp. 996-997.
- Whitten K.W., Gailey K.D. and Davis R.E. "General Chemistry" (4th ed., Saunders College Publishing 1992), p. 976-7 ISBN 978-0-03-072373-5
- Ernest L. Eliel and Samuel H. Wilen (1994). Stereochemistry of Organic Compounds. Wiley Interscience. p. 1203.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "optical isomers". doi:10.1351/goldbook.O04308
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "isomerization". doi:10.1351/goldbook.I03295
- Brown, K. N.; Field, L. D.; Lay, P. A.; Lindall, C. M.; Masters, A. F. (1990). "(η5-Pentaphenylcyclopentadienyl){1-(η6-phenyl)-2,3,4,5-tetraphenylcyclopentadienyl}iron(II), [Fe(η5-C5Ph5){(η6-C6H5)C5Ph4}], a linkage isomer of decaphenylferrocene". J. Chem. Soc., Chem. Commun. (5): 408–410. doi:10.1039/C39900000408.
- Field, L. D.; Hambley, T. W.; Humphrey, P. A.; Lindall, C. M.; Gainsford, G. J.; Masters, A. F.; Stpierre, T. G.; Webb, J. (1995). "Decaphenylferrocene". Aust. J. Chem. 48 (4): 851–860. doi:10.1071/CH9950851.
- F. Kurzer (2000). "Fulminic Acid in the History of Organic Chemistry". J. Chem. Educ. 77 (7): 851–857. Bibcode:2000JChEd..77..851K. doi:10.1021/ed077p851.
- Esteban, Soledad. (2008). "Liebig–Wöhler Controversy and the Concept of Isomerism". J. Chem. Educ. 85 (9): 1201. Bibcode:2008JChEd..85.1201E. doi:10.1021/ed085p1201.
- L. Pasteur (1848) "Mémoire sur la relation qui peut exister entre la forme cristalline et la composition chimique, et sur la cause de la polarisation rotatoire" (Memoir on the relationship which can exist between crystalline form and chemical composition, and on the cause of rotary polarization)," Comptes rendus de l'Académie des sciences (Paris), vol. 26, pages 535–538.
- L. Pasteur (1848) "Sur les relations qui peuvent exister entre la forme cristalline, la composition chimique et le sens de la polarisation rotatoire" (On the relations that can exist between crystalline form, chemical composition, and the sense of rotary polarization), Annales de Chimie et de Physique, 3rd series, vol. 24, no. 6, pages 442–459.
- See: Jac. Berzelius (1830) “Om sammansättningen af vinsyra och drufsyra (John’s säure aus den Voghesen), om blyoxidens atomvigt, samt allmänna anmärkningar om sådana kroppar som hafva lika sammansättning, men skiljaktiga egenskaper” (On the composition of tartaric acid and racemic acid (John's acid of the Vosges), on the molecular weight of lead oxide, together with general observations on those bodies that have the same composition but distinct properties) Kongliga Svenska Vetenskaps Academiens Handling (Transactions of the Royal Swedish Science Academy), vol. 49, pages 49–80; see especially page 70. Reprinted in German in: J.J. Berzelius (1831) “Über die Zusammensetzung der Weinsäure und Traubensäure (John's säure aus den Voghesen), über das Atomengewicht des Bleioxyds, nebst allgemeinen Bemerkungen über solche Körper, die gleiche Zusammensetzung, aber ungleiche Eigenschaften besitzen," Annalen der Physik und Chemie, vol. 19, pages 305–335; see especially page 326. Reprinted in French in: J.J. Berzelius (1831) “Composition de l’acide tartarique et de l’acide racémique (traubensäure); poids atomique de l’oxide de plomb, et remarques générals sur les corps qui ont la même composition, et possèdent des proprietés différentes,” Annales de Chimie et de Physique, vol. 46, pages 113–147; see especially page 136.
External links
| Wikiquote has quotations related to: Isomer |
| Wikimedia Commons has media related to Isomerism. |