Intrinsically photosensitive retinal ganglion cells
Intrinsically photosensitive retinal ganglion cells (ipRGCs), also called photosensitive retinal ganglion cells (pRGC), or melanopsin-containing retinal ganglion cells (mRGCs), are a type of neuron in the retina of the mammalian eye. The presence of ipRGCs were first noted in 1923 when rodless, coneless mice still responded to a light stimulus through pupil constriction, suggesting that rods and cones are not the only light-sensitive neurons in the retina. It wasn't until the 1980s that advancements in research on these cells began. Recent research has shown that these retinal ganglion cells, unlike other retinal ganglion cells, are intrinsically photosensitive due to the presence of melanopsin, a light-sensitive protein. Therefore they constitute a third class of photoreceptors, in addition to rod and cone cells.[1]
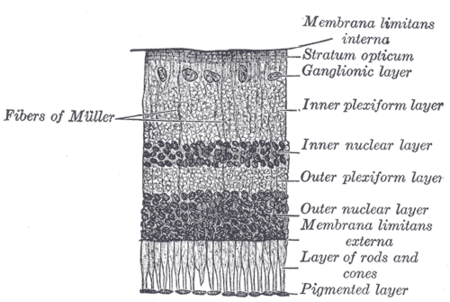
Overview
Compared to the rods and cones, the ipRGCs respond more sluggishly and signal the presence of light over the long term.[2] They represent a very small subset (~1%) of the retinal ganglion cells.[3] Their functional roles are non-image-forming and fundamentally different from those of pattern vision; they provide a stable representation of ambient light intensity. They have at least three primary functions:
- They play a major role in synchronizing circadian rhythms to the 24-hour light/dark cycle, providing primarily length-of-day and length-of-night information. They send light information via the retinohypothalamic tract (RHT) directly to the circadian pacemaker of the brain, the suprachiasmatic nucleus of the hypothalamus. The physiological properties of these ganglion cells match known properties of the daily light entrainment (synchronization) mechanism regulating circadian rhythms. In addition, ipRGCs could also influence peripheral tissues such as the hair follicle regeneration through SCN-sympathetic nerve circuit.[4]
- Photosensitive ganglion cells innervate other brain targets, such as the center of pupillary control, the olivary pretectal nucleus of the midbrain. They contribute to the regulation of pupil size and other behavioral responses to ambient lighting conditions.[5]
- They contribute to photic regulation and acute photic suppression of release of the hormone melatonin.[5]
- In rats, they play some role in conscious visual perception, including perception of regular gratings, light levels, and spatial information.[5]
Photoreceptive ganglion cells have been isolated in humans, where, in addition to regulating the circadian rhythm, they have been shown to mediate a degree of light recognition in rodless, coneless subjects suffering with disorders of rod and cone photoreceptors.[6] Work by Farhan H. Zaidi and colleagues showed that photoreceptive ganglion cells may have some visual function in humans.
The photopigment of photoreceptive ganglion cells, melanopsin, is excited by light mainly in the blue portion of the visible spectrum (absorption peaks at ~480 nanometers[7]). The phototransduction mechanism in these cells is not fully understood, but seems likely to resemble that in invertebrate rhabdomeric photoreceptors. In addition to responding directly to light, these cells may receive excitatory and inhibitory influences from rods and cones by way of synaptic connections in the retina.
The axons from these ganglia innervate regions of the brain related to object recognition, including the superior colliculus and dorsal lateral geniculate nucleus.[5]
Structure
ipRGC receptor
These photoreceptor cells project both throughout the retina and into the brain. They contain the photopigment melanopsin in varying quantities along the cell membrane, including on the axons up to the optic disc, the soma, and dendrites of the cell.[1] ipRGCs contain membrane receptors for the neurotransmitters glutamate, glycine, and GABA.[8] Photosensitive ganglion cells respond to light by depolarizing, thus increasing the rate at which they fire nerve impulses, which is opposite to that of other photoreceptor cells, which hyperpolarize in response to light.[9]
Results of studies in mice suggest that the axons of ipRGCs are unmyelinated.[1]
Melanopsin
Unlike other photoreceptor pigments, melanopsin has the ability to act as both the excitable photopigment and as a photoisomerase. Instead of requiring additional cells to revert between the two isoforms, from all-trans-retinal back into 11-cis-retinal before it can undergo another phototransduction, like the photoreceptor cones, which rely on Müller cells and retinal pigment epithelium cells for this conversion, melanopsin is able to isomerize all-trans-retinal into 11-cis-retinal when stimulated with light without help from additional cells.[8] The two isoforms of melanopsin differ in their spectral sensitivity, for the 11-cis-retinal isoform is more responsive to shorter wavelengths of light, while the all-trans isoform is more responsive to longer wavelengths of light.[10]
Synaptic inputs and outputs
Inputs
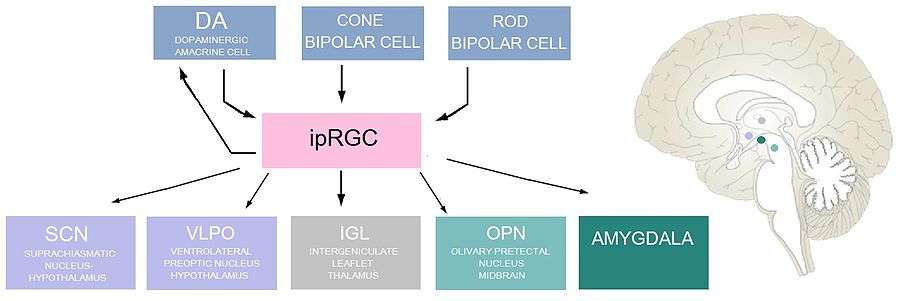
ipRGCs are both pre- and postsynaptic to dopaminergic amacrine cells (DA cells) via reciprocal synapses, with ipRGCs sending excitatory signals to the DA cells, and the DA cells sending inhibitory signals to the ipRGCs. These inhibitory signals are mediated through GABA, which is co-released from the DA cells along with dopamine. Dopamine has functions in the light-adaptation process by up-regulating melanopsin transcription in ipRGCs and thus increasing the photoreceptor's sensitivity.[1] In parallel with the DA amacrine cell inhibition, somatostatin-releasing amacrine cells, themselves inhibited by DA amacrine cells, inhibit ipRGCs.[11] Other synaptic inputs to ipRGC dendrites include cone bipolar cells and rod bipolar cells.[8]
Outputs
One postsynaptic target of ipRGCs is the suprachiasmatic nucleus (SCN) of the hypothalamus, which serves as the circadian clock in an organism. ipRGCs release both pituitary adenylyl cyclase-activating protein (PACAP) and glutamate onto the SCN via a monosynaptic connection called the retinohypothalamic tract (RHT).[12] Glutamate has an excitatory effect on SCN neurons, and PACAP appears to enhance the effects of glutamate in the hypothalamus.[13]
Other post synaptic targets of ipRGCs include: the intergenticulate leaflet (IGL), a cluster of neurons located in the thalamus, which play a role in circadian entrainment; the olivary pretectal nucleus (OPN), a cluster of neurons in the midbrain that controls the pupillary light reflex; the ventrolateral preoptic nucleus (VLPO), located in the hypothalamus and is a control center for sleep; as well as to the amygdala.[1]
Function
Pupillary light reflex
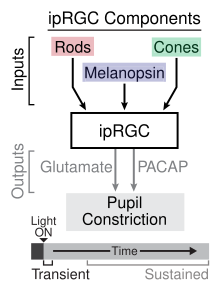
Using various photoreceptor knockout mice, researchers have identified the role of ipRGCs in both the transient and sustained signaling of the pupillary light reflex (PLR).[14] Transient PLR occurs at dim to moderate light intensities and is a result of phototransduction occurring in rod cells, which provide synaptic input onto ipRGCs, which in turn relay the information to the olivary pretectal nucleus in the midbrain.[15] The neurotransmitter involved in the relay of information to the midbrain from the ipRGCs in the transient PLR is glutamate. At brighter light intensities the sustained PLR occurs, which involves both phototransduction of the rod providing input to the ipRGCs and phototransduction of the ipRGCs themselves via melanopsin. Researchers have suggested that the role of melanopsin in the sustained PLR is due to its lack of adaptation to light stimuli in contrast to rod cells, which exhibit adaptation. The sustained PLR is maintained by PACAP release from ipRGCs in a pulsatile manner.[14]
Possible role in conscious sight
Experiments with rodless, coneless humans allowed another possible role for the receptor to be studied. In 2007, a new role was found for the photoreceptive ganglion cell. Zaidi and colleagues showed that in humans the retinal ganglion cell photoreceptor contributes to conscious sight as well as to non-image-forming functions like circadian rhythms, behaviour and pupillary reactions.[6] Since these cells respond mostly to blue light, it has been suggested that they have a role in mesopic vision and that the old theory of a purely duplex retina with rod (dark) and cone (light) light vision was simplistic. Zaidi and colleagues' work with rodless, coneless human subjects hence has also opened the door into image-forming (visual) roles for the ganglion cell photoreceptor.
The discovery that there are parallel pathways for vision was made: one classic rod- and cone-based arising from the outer retina, the other a rudimentary visual brightness detector arising from the inner retina. The latter seems to be activated by light before the former.[6] Classic photoreceptors also feed into the novel photoreceptor system, and colour constancy may be an important role as suggested by Foster.
It has been suggested by the authors of the rodless, coneless human model that the receptor could be instrumental in understanding many diseases, including major causes of blindness worldwide such as glaucoma, a disease which affects ganglion cells.
In other mammals, photosensitive ganglia have proven to have a genuine role in conscious vision. Tests conducted by Jennifer Ecker et al. found that rats lacking rods and cones were able to learn to swim toward sequences of vertical bars rather than an equally luminescent gray screen.[5]
Violet-to-blue light
Most work suggests that the peak spectral sensitivity of the receptor is between 460 and 484 nm. Lockley et al. in 2003[16] showed that 460 nm (blue) wavelengths of light suppress melatonin twice as much as 555 nm (green) light, the peak sensitivity of the photopic visual system. In work by Zaidi, Lockley and co-authors using a rodless, coneless human, it was found that a very intense 481 nm stimulus led to some conscious light perception, meaning that some rudimentary vision was realized.[6]
Discovery
In 1923, Clyde E. Keeler observed that the pupils in the eyes of blind mice he had accidentally bred still responded to light.[17] The ability of the rodless, coneless mice to retain a pupillary light reflex was suggestive of an additional photoreceptor cell.[8]
In the 1980s, research in rod- and cone-deficient rats showed regulation of dopamine in the retina, a known neuromodulator for light adaptation and photoentrainment.[1]
Research continued in 1991, when Russell G. Foster and colleagues, including Ignacio Provencio, showed that rods and cones were not necessary for photoentrainment, the visual drive of the circadian rhythm, nor for the regulation of melatonin secretion from the pineal gland, via rod- and cone-knockout mice.[18][8] Later work by Provencio and colleagues showed that this photoresponse was mediated by the photopigment melanopsin, present in the ganglion cell layer of the retina.[19]
The photoreceptors were identified in 2002 by Samer Hattar, David Berson and colleagues, where they were shown to be melanopsin expressing ganglion cells that possessed an intrinsic light response and projected to a number of brain areas involved in non-image-forming vision.[20][21]
In 2005, Panda, Melyan, Qiu, and colleagues demonstrated that the melanopsin photopigment was the phototransduction pigment in ganglion cells.[22][23] Dennis Dacey and colleagues showed in a species of Old World monkey that giant ganglion cells expressing melanopsin projected to the lateral geniculate nucleus (LGN).[24][3] Previously only projections to the midbrain (pre-tectal nucleus) and hypothalamus (supra-chiasmatic nuclei, SCN) had been shown. However, a visual role for the receptor was still unsuspected and unproven.
Research
Research in humans
Attempts were made to hunt down the receptor in humans, but humans posed special challenges and demanded a new model. Unlike in other animals, researchers could not ethically induce rod and cone loss either genetically or with chemicals so as to directly study the ganglion cells. For many years, only inferences could be drawn about the receptor in humans, though these were at times pertinent.
In 2007, Zaidi and colleagues published their work on rodless, coneless humans, showing that these people retain normal responses to nonvisual effects of light.[6][25] The identity of the non-rod, non-cone photoreceptor in humans was found to be a ganglion cell in the inner retina as shown previously in rodless, coneless models in some other mammals. The work was done using patients with rare diseases that wiped out classic rod and cone photoreceptor function but preserved ganglion cell function.[6][25] Despite having no rods or cones, the patients continued to exhibit circadian photoentrainment, circadian behavioural patterns, melatonin suppression, and pupil reactions, with peak spectral sensitivities to environmental and experimental light that match the melanopsin photopigment. Their brains could also associate vision with light of this frequency. Clinicians and scientists are now seeking to understand the new receptor's role in human diseases and blindness.[26] Intrinsically photosensitive RGCs have also been implicated in the exacerbation of headache by light during migraine attacks. [27]
References
- Do MT, Yau KW (October 2010). "Intrinsically photosensitive retinal ganglion cells". Physiological Reviews. 90 (4): 1547–81. doi:10.1152/physrev.00013.2010. PMC 4374737. PMID 20959623.
- Wong KY, Dunn FA, Berson DM (December 2005). "Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells". Neuron. 48 (6): 1001–10. doi:10.1016/j.neuron.2005.11.016. PMID 16364903.
- Berson DM (June 2003). "Strange vision: ganglion cells as circadian photoreceptors". Trends in Neurosciences. 26 (6): 314–20. doi:10.1016/S0166-2236(03)00130-9. PMID 12798601. S2CID 15149809.
- Fan SM, Chang YT, Chen CL, Wang WH, Pan MK, Chen WP, et al. (July 2018). "External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway". Proceedings of the National Academy of Sciences of the United States of America. 115 (29): E6880–E6889. doi:10.1073/pnas.1719548115. PMC 6055137. PMID 29959210.
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, et al. (July 2010). "Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision". Neuron. 67 (1): 49–60. doi:10.1016/j.neuron.2010.05.023. PMC 2904318. PMID 20624591.
- Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, et al. (December 2007). "Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina". Current Biology. 17 (24): 2122–8. doi:10.1016/j.cub.2007.11.034. PMC 2151130. PMID 18082405. Lay summary – Cell Press (December 13, 2007).
- Berson DM (August 2007). "Phototransduction in ganglion-cell photoreceptors". Pflugers Archiv. 454 (5): 849–55. doi:10.1007/s00424-007-0242-2. PMID 17351786.
- Kolb H, Fernandez E, Nelson R (1995-01-01). Kolb H, Fernandez E, Nelson R (eds.). Webvision: The Organization of the Retina and Visual System. University of Utah Health Sciences Center. PMID 21413389.
- Do MT, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, Yau KW (January 2009). "Photon capture and signalling by melanopsin retinal ganglion cells". Nature. 457 (7227): 281–7. Bibcode:2009Natur.457..281D. doi:10.1038/nature07682. PMC 2794210. PMID 19118382.
- Chellappa SL, Ly JQ, Meyer C, Balteau E, Degueldre C, Luxen A, et al. (April 2014). "Photic memory for executive brain responses". Proceedings of the National Academy of Sciences of the United States of America. 111 (16): 6087–91. Bibcode:2014PNAS..111.6087C. doi:10.1073/pnas.1320005111. PMC 4000819. PMID 24616488.
- Vuong HE, Hardi CN, Barnes S, Brecha NC (December 2015). "Parallel Inhibition of Dopamine Amacrine Cells and Intrinsically Photosensitive Retinal Ganglion Cells in a Non-Image-Forming Visual Circuit of the Mouse Retina". The Journal of Neuroscience. 35 (48): 15955–70. doi:10.1523/jneurosci.3382-15.2015. PMC 4666919. PMID 26631476.
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelièvre V, et al. (November 2004). "Selective deficits in the circadian light response in mice lacking PACAP". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 287 (5): R1194-201. doi:10.1152/ajpregu.00268.2004. PMID 15217792.
- Butcher GQ, Lee B, Cheng HY, Obrietan K (June 2005). "Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism". The Journal of Neuroscience. 25 (22): 5305–13. doi:10.1523/jneurosci.4361-04.2005. PMC 6724997. PMID 15930378.
- Keenan WT, Rupp AC, Ross RA, Somasundaram P, Hiriyanna S, Wu Z, et al. (September 2016). "A visual circuit uses complementary mechanisms to support transient and sustained pupil constriction". eLife. 5. doi:10.7554/eLife.15392. PMC 5079752. PMID 27669145.
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM (March 2007). "Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells". Vision Research. 47 (7): 946–54. doi:10.1016/j.visres.2006.12.015. PMC 1945238. PMID 17320141.
- Lockley SW, Brainard GC, Czeisler CA (September 2003). "High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light". The Journal of Clinical Endocrinology and Metabolism. 88 (9): 4502–5. doi:10.1210/jc.2003-030570. PMID 12970330.
- Keeler CE (October 1928). "Blind Mice". Journal of Experimental Zoology. 51 (4): 495–508. doi:10.1002/jez.1400510404.
- Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M (July 1991). "Circadian photoreception in the retinally degenerate mouse (rd/rd)". Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology. 169 (1): 39–50. doi:10.1007/BF00198171. PMID 1941717. S2CID 1124159.
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD (January 2000). "A novel human opsin in the inner retina". The Journal of Neuroscience. 20 (2): 600–5. doi:10.1523/jneurosci.20-02-00600.2000. PMC 6772411. PMID 10632589.
- Berson DM, Dunn FA, Takao M (February 2002). "Phototransduction by retinal ganglion cells that set the circadian clock". Science. 295 (5557): 1070–3. Bibcode:2002Sci...295.1070B. doi:10.1126/science.1067262. PMID 11834835. S2CID 30745140.
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW (February 2002). "Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity". Science. 295 (5557): 1065–70. Bibcode:2002Sci...295.1065H. doi:10.1126/science.1069609. PMC 2885915. PMID 11834834.
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T (January 2005). "Illumination of the melanopsin signaling pathway". Science. 307 (5709): 600–4. Bibcode:2005Sci...307..600P. doi:10.1126/science.1105121. PMID 15681390. S2CID 22713904.
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM (February 2005). "Induction of photosensitivity by heterologous expression of melanopsin". Nature. 433 (7027): 745–9. Bibcode:2005Natur.433..745Q. doi:10.1038/nature03345. PMID 15674243. S2CID 24999816.
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, et al. (February 2005). "Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN". Nature. 433 (7027): 749–54. Bibcode:2005Natur.433..749D. doi:10.1038/nature03387. PMID 15716953. S2CID 4401722.
- Coghlan A (2007). "How blind people see sunrise and sunset". New Scientist. 196 (2635–2636): 9. doi:10.1016/S0262-4079(07)63172-8.
- Schor J (2008-04-19). "Blue Light and Melatonin" (web page). Morning Light. Retrieved 2008-05-30.
- Noseda R (2010). "A neural mechanism for exacerbation of headache by light". Nature Neuroscience. 13: 239–245. doi:10.1038/nn.2475.

