Ommatidium
The compound eyes of arthropods like insects, crustaceans and millipedes[1] are composed of units called ommatidia (singular: ommatidium). An ommatidium contains a cluster of photoreceptor cells surrounded by support cells and pigment cells. The outer part of the ommatidium is overlaid with a transparent cornea. Each ommatidium is innervated by one axon bundle (usually consisting of 6–9 axons, depending on the number of rhabdomeres)[2]:162 and provides the brain with one picture element. The brain forms an image from these independent picture elements. The number of ommatidia in the eye depends upon the type of arthropod and range from as low as 5 as in the Antarctic isopod Glyptonotus antarcticus,[3] or a handful in the primitive Zygentoma, to around 30,000 in larger Anisoptera dragonflies and some Sphingidae moths.[4]
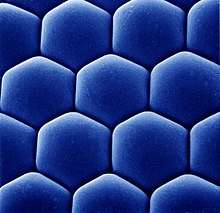
Ommatidia are typically hexagonal in cross-section and approximately ten times longer than wide. The diameter is largest at the surface, tapering toward the inner end. At the outer surface, there is a cornea, below which is a pseudocone that acts to further focus the light. The cornea and pseudocone form the outer ten percent of the length of the ommatidium.
The specific composition of ommatidium varies between different organisms. The butterfly compound eye consists of multiple eye units, or ommatidia. Each ommatidium consists of nine photoreceptor cells (numbered from R1–R9), primary and secondary pigment cells.[5] Nymphalid butterflies have the simplest eye ommatidium structure, consisting of eight photoreceptor cells (R1–R8) and a tiny R9 cell organized into a different tier. [5] These "R cells" tightly pack the ommatidium. The portion of the R cells at the central axis of the ommatidium collectively form a light guide, a transparent tube, called the rhabdom.
Although composed of over 16,000 cells,[6] the Drosophila compound eye is a simple repetitive pattern of 700 to 750 of ommatidia,[7] initiated in the larval eye imaginal disc. Each ommatidium consists of 14 neighboring cells: 8 photoreceptor neurons in the core, 4 non-neuronal cone cells and 2 primary pigment cells.[6] A hexagonal lattice of pigment cells insulates the ommatidial core from neighboring ommatidia to optimize coverage of the visual field, which therefore affects the acuity of Drosophila vision[7].
In true flies, the rhabdom has separated into seven independent rhabdomeres (there are actually eight, but the two central rhabdomeres responsible for color vision sit one atop the other), such that a small inverted 7-pixel image is formed in each ommatidium. Simultaneously, the rhabdomeres in adjacent ommatidia are aligned such that the field of view within an ommatidium is the same as that between ommatidia. The advantage of this arrangement is that the same visual axis is sampled from a larger area of the eye, thereby increasing sensitivity by a factor of seven, without increasing the size of the eye or reducing its acuity. Achieving this has also required the rewiring of the eye such that the axon bundles are twisted through 180 degrees (re-inverted), and each rhabdomere is united with those from the six adjacent ommatidia that share the same visual axis. Thus, at the level of the lamina – the first optical processing center of the insect brain – the signals are input in exactly the same manner as in the case of a normal apposition compound eye, but the image is enhanced. This visual arrangement is known as neural superposition.[2]:163–4
Since an image from the compound eye is created from the independent picture elements produced by ommatidia, it is important for the ommatidia to react only to that part of the scene directly in front of them. To prevent light entering at an angle from being detected by the ommatidium it entered, or by any of the neighboring ommatidia, six pigment cells are present. The pigment cells line the outside of each ommatidium. Each pigment cell is situated at the apex of the hexagons and thus lines the outside of three ommatidia. Light entering at an angle passes through the thin cross-section of the photoreceptor cell, with only a tiny chance of exciting it, and is absorbed by the pigment cell, before it can enter a neighboring ommatidium. In many species, in low-light situations, the pigment is withdrawn, so that light entering the eye might be detected by any of several ommatidia. This enhances light detection but lowers resolution.
The size of the ommatidia varies according to species, but ranges from 5 to 50 micrometres. The rhabdoms within them may cross-section at least as small as 1.x micrometres, the category of "small" being assigned in some cross-species studies to those under 2 micrometers.[8] A microlens array can be seen as an elementary, biomimetic analogy of ommatidia.
See also
- Pseudopupil
- Arthropod eye
- Apposition eye
- Superposition eye
References
- Müller CH, Sombke A, Rosenberg J (December 2007). "The fine structure of the eyes of some bristly millipedes (Penicillata, Diplopoda): additional support for the homology of mandibulate ommatidia". Arthropod Structure & Development. 36 (4): 463–76. doi:10.1016/j.asd.2007.09.002. PMID 18089122.
- Land MF, Nilsson D (2012). Animal Eyes (Second ed.). Oxford University Press. ISBN 978-0-19-958114-6.
- Meyer-Rochow VB (1982). "The divided eye of the isopod Glyptonotus antarcticus: effects of unilateral dark adaptation and temperature elevation". Proceedings of the Royal Society of London. B215 (1201): 433–450. Bibcode:1982RSPSB.215..433M. doi:10.1098/rspb.1982.0052.
- Common IF (1990). Moths of Australia. Brill. p. 15. ISBN 978-90-04-09227-3.
- Briscoe AD (June 2008). "Reconstructing the ancestral butterfly eye: focus on the opsins". The Journal of Experimental Biology. 211 (Pt 11): 1805–13. doi:10.1242/jeb.013045. PMID 18490396.
- Cagan R (2009). "Principles of Drosophila eye differentiation". Current Topics in Developmental Biology. 89. Elsevier: 115–35. doi:10.1016/s0070-2153(09)89005-4. ISBN 9780123749024. PMC 2890271. PMID 19737644. Cite journal requires
|journal=(help) - Cagan RL, Ready DF (December 1989). "The emergence of order in the Drosophila pupal retina". Developmental Biology. 136 (2): 346–62. doi:10.1016/0012-1606(89)90261-3. PMID 2511048.
- Land MF (1997). "Visual acuity in insects". Annual Review of Entomology. 42: 147–77. doi:10.1146/annurev.ento.42.1.147. PMID 15012311.
- Li X, Carthew RW (December 2005). "A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye". Cell. 123 (7): 1267–77. doi:10.1016/j.cell.2005.10.040. PMID 16377567.
.jpg)