Acetylenedicarboxylic acid
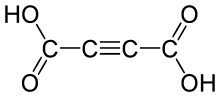
Acetylenedicarboxylic acid or butynedioic acid is an organic compound (a dicarboxylic acid) with the formula C4H2O4 or HO
2CC≡CCO
2H. It is a crystalline solid that is soluble in diethyl ether.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
But-2-ynedioic acid | |
| Other names
2-Butynedioic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 878357 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.033 |
| EC Number |
|
| 26624 | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H2O4 | |
| Molar mass | 114.056 g·mol−1 |
| Appearance | Crystalline solid |
| Melting point | 175 to 176 °C (347 to 349 °F; 448 to 449 K) (decomposes)[2] 180–187 °C (decomposes)[1] |
| Conjugate base | Hydrogenacetylenedicarboxylate (chemical formula HC4O4−) |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H301, H314, H315, H319, H335 |
| P260, P261, P264, P270, P271, P280, P301+310, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P330, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The removal of two protons yields the acetylenedicarboxylate dianion C
4O2−
4, which consists only of carbon and oxygen, making it an oxocarbon anion. Partial ionization yields the monovalent hydrogenacetylenedicarboxylate anion HC
4O−
4.
The acid was first described in 1877 by Polish chemist Ernest Bandrowski.[2][3][4] It can be obtained by treating α,β-dibromosuccinic acid with potassium hydroxide KOH in methanol or ethanol. The reaction yields potassium bromide and potassium acetylenedicarboxylate. The salts are separated and the latter is treated with sulfuric acid.[2]
Acetylenedicarboxylic acid is used in the synthesis of dimethyl acetylenedicarboxylate, an important laboratory reagent. The acid is commonly traded as a laboratory chemical. It can also be reacted with sulfur tetrafluoride to produce hexafluoro-2-butyne, a powerful dienophile for use in Diels-Alder reactions.
Fatty alcohol esters of acetylenedicarboxylic acid can be used for the preparation of phase change materials (PCM) [5].
Anions and salts
Hydrogenacetylenedicarboxylate (often abbreviated as Hadc or HADC) is a monovalent anion of acetylenedicarboxylic acid with the formula C
4HO−
4 or HO
2CC≡CCO−
2. The anion can be derived from acetylenedicarboxylic acid by removal of a single proton or from the acetylenedicarboxylate dianion by addition of a proton. The name is also used for any salt of this anion. Salts of this anion are of interest in crystallography because they contain unusually short and strong hydrogen bonds. In many crystalline salts (with the exception of the lithium one), the HADC units form linear chains connected by strong hydrogen bonds. Each carboxylate group is usually planar; but the two groups may lie in different planes due to rotation about the carbon–carbon bonds. They are coplanar in the hydrated salts NaHC
4O
4·2H2O and CsHC
4O
4·2H2O, nearly coplanar in the guanidinium salt C(NH
2)+
3·C
4HO−
4, but off by 60° or more in other salts such as anhydrous KHC
4O
4.[6]
Potassium hydrogenacetylenedicarboxylate is a potassium salt of HADC with chemical formula KC4HO4 or K+
·HC
4O−
4, often abbreviated as KHadc. It is often called potassium hydrogen acetylenedicarboxylate or monopotassium acetylenedicarboxylate. The salt can be obtained from acetylenedicarboxylic acid and is a common laboratory starting material for the synthesis of other derivatives of that acid. In the crystalline form, the hydrogenacetylenedicarboxylate anions are joined into linear chains by uncommonly short hydrogen bonds.[7][8]

Acetylenedicarboxylate (often abbreviated as ADC or adc) is a divalent anion with formula C
4O2−
4 or [O2C–C≡C–CO2]2−; or any salt or ester thereof. The anion can be derived from acetylenedicarboxylic acid by the loss of two protons. It is one of several oxocarbon anions which, like carbonate CO2−
3 and oxalate C
2O2−
4, consist solely of carbon and oxygen. The ADC anion can at as a ligand in organometallic complexes, such as the blue polymeric complex with copper(II) and 2,2′-bipyridine, [Cu2+·C
4O2−
4·(C
5H
4N)
2]
n.[9][10] Thallium(I) acetylenedicarboxylate (Tl2C4O4) decomposes at 195 °C, leaving a residue of pyrophoric thallium powder.[11]
References
- "Acetylenedicarboxylic acid". Sigma-Aldrich.
- Abbott, T. W.; Arnold, R. T.; Thompson, R. B. "Acetylenedicarboxylic acid". Organic Syntheses.; Collective Volume, 2, p. 10
- Bandrowski, E. (1877). "Ueber Acetylendicarbonsäure" [On acetylenedicarboxylic acid]. Berichte der Deutschen Chemischen Gesellschaft. 10: 838–842. doi:10.1002/cber.187701001231.
- E. Bandrowski (1879). "Weitere Beiträge zur Kenntniss der Acetylendicarbonsäure" [Further comments on the description of acetylenedicarboxylic acid]. Berichte der Deutschen Chemischen Gesellschaft. 12 (2): 2212–2216. doi:10.1002/cber.187901202261.
- Daglar, Ozgun; Çakmakçı, Emrah; Hizal, Gurkan; Tunca, Umit; Durmaz, Hakan (2020-05-05). "Extremely fast synthesis of polythioether based phase change materials (PCMs) for thermal energy storage". European Polymer Journal. 130: 109681. doi:10.1016/j.eurpolymj.2020.109681. ISSN 0014-3057.
- Leban, I; Rupnik, A (1992). "Structure of guanidinium hydrogen acetylenedicarboxylate, CH
6N+
3·C
4HO4−
". Acta Crystallographica Section C. 48 (5): 821. doi:10.1107/S010827019101154X. - Leban, Ivan; Golič, Ljubo; Speakman, J. Clare (1973). "Crystal structures of the acid salts of some dibasic acids. Part VII. An X-ray study of potassium hydrogen acetylenedicarboxylate: The α-form". J. Chem. Soc., Perkin Trans. 2 (6): 703–705. doi:10.1039/P29730000703.
- Miyakubo, Keisuke (1994). Nuclear magnetic resonance studies of dynamical structure of one-dimensional hydrogen-bonded system in the acid salts of some dicarboxylic acids (PDF) (Ph.D.). Osaka University.
- Li, Ming-xing; Shao, Min; Dai, Hui; An, Bao-li; Lu, Wen-cong; Zhu, Yu; Du, Chen-xia (2005). "Synthesis and Crystal Structure of a Novel Copper(II) Complex with Acetylenedicarboxylate and 2,2′-Bipyridine". Chinese Chemical Letters. 16 (10): 1405–1408.
- Shao, Min; Li, Ming-xing; Dai, Hui; Lu, Wen-cong; An, Bao-li (2007). "Polynuclear complexes incorporating Cu(II) and Mn(II) centers bridged by acetylenedicarboxylate: Structure, thermal stability and magnetism". Journal of Molecular Structure. 829 (1–3): 155–160.
- Ahlers, Ruth; Ruschewitz, Uwe (2009). "Non-centrosymmetric coordination polymers based on thallium and acetylenedicarboxylate". Solid State Sciences. 11 (6): 1058–1064.