Hexamethylphosphoramide
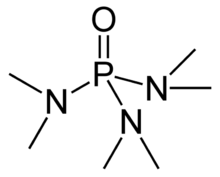
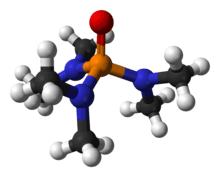
Hexamethylphosphoramide, often abbreviated HMPA, is a phosphoramide (an amide of phosphoric acid) with the formula [(CH3)2N]3PO. This colorless liquid is a useful reagent in organic synthesis.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Hexamethylphosphoric triamide[1] | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.595 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H18N3OP | |
| Molar mass | 179.20 g/mol |
| Appearance | clear, colorless liquid[2] |
| Odor | aromatic, mild, amine-like[2] |
| Density | 1.03 g/cm3 |
| Melting point | 7.20 °C (44.96 °F; 280.35 K) |
| Boiling point | 232.5 °C (450.5 °F; 505.6 K) CRC[3] |
| miscible[2] | |
| Vapor pressure | 0.03 mmHg (4.0 Pa) at 20 °C[2] |
| Hazards | |
| Main hazards | Suspected Carcinogen[2] |
| Safety data sheet | Oxford MSDS |
| Flash point | 104.4 °C (219.9 °F; 377.5 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[2] |
REL (Recommended) |
Ca[2] |
IDLH (Immediate danger) |
Ca [N.D.][2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure and reactivity
HMPA is the oxide of the highly basic tertiary phosphine hexamethylphosphorous triamide (HMPT[note 1]), P(NMe2)3. Like other phosphine oxides (such as triphenylphosphine oxide), the molecule has a tetrahedral core and a P=O bond that is highly polarized, with significant negative charge residing on the oxygen atom.
Compounds containing a nitrogen–phosphorus bond typically are degraded by hydrochloric acid to form a protonated amine and phosphate.
It dissolves alkali metals forming blue solutions which are stable for a few hours.[4] Solvated electrons are present in these blue solutions.[5]
Applications
HMPA is a specialty solvent for polymers, gases, and organometallic compounds. It improves the selectivity of lithiation reactions by breaking up the oligomers of lithium bases such as butyllithium. Because HMPA selectively solvates cations, it accelerates otherwise slow SN2 reactions by generating more bare anions. The basic nitrogen centers in HMPA coordinate strongly to Li+.[6]
HMPA is a ligand in the useful reagents based on molybdenum peroxide complexes, for example, MoO(O2)2(HMPA)(H2O) is used as an oxidant in organic synthesis.[7]
Alternative reagents
Dimethyl sulfoxide can often be used in place of HMPA as a cosolvent. Both are strong hydrogen bond acceptors, and their oxygen atoms bind metal cations. Other alternatives to HMPA include the N,N′-tetraalkylureas DMPU (dimethylpropyleneurea)[8][9] or DMI (1,3-dimethyl-2-imidazolidinone).[10] Tripyrrolidinophosphoric acid triamide (TPPA) has been reported to be a good substitute reagent for HMPA in reductions with samarium diiodide[11] and as a Lewis base additive to many reactions involving samarium ketyls.[12]
Toxicity
HMPA is only mildly toxic but has been shown to cause cancer in rats.[6] HMPA can be degraded by the action of hydrochloric acid.
Notes
- Confusingly, some sources (such as e-EROS) list HMPT as an abbreviation for O=P(NMe2)3 (hexamethylphosphoric triamide), as well as an abbreviation for P(NMe2)3 (hexamethylphosphorous triamide).
References
- "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. P001–P004. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- NIOSH Pocket Guide to Chemical Hazards. "#0321". National Institute for Occupational Safety and Health (NIOSH).
- Haynes, William M. (2010). Handbook of Chemistry and Physics (91 ed.). Boca Raton, Florida: CRC Press. p. 3-280. ISBN 978-1439820773.
- Luehrs, Dean C.; Kohut, John P. (1974). "Hexamethylphosphoramide solvates of alkali metal salts". Journal of Inorganic and Nuclear Chemistry. 36 (7): 1459–1460. doi:10.1016/0022-1902(74)80605-6.
- Gremmo, Norberto; Randles, John E. B. (1974). "Solvated electrons in hexamethylphosphoramide. Part 1.—Conductivity of solutions of alkali metals". Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases. 70: 1480–1487. doi:10.1039/F19747001480.
- Dykstra, R. R. (2001). "Hexamethylphosphoric Triamide". Encyclopedia of Reagents for Organic Synthesis. New York, NY: John Wiley & Sons. doi:10.1002/047084289X.rh020. ISBN 978-0471936237.
- Dickman, Michael H.; Pope, Michael T. (1994). "Peroxo and Superoxo Complexes of Chromium, Molybdenum, and Tungsten". Chemical Reviews. 94 (3): 569–584. doi:10.1021/cr00027a002.
- Mukhopadhyay, T.; Seebach, D. (1982). "Substitution of HMPT by the Cyclic Urea DMPU as a Cosolvent for highly Reactive Nucleophiles and Bases". Helvetica Chimica Acta. 65 (1): 385–391. doi:10.1002/hlca.19820650141.
- Beck, A. K.; Seebach, D. (2001). "N,N′-Dimethylpropyleneurea". Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley & Sons. doi:10.1002/047084289X.rd366. ISBN 978-0471936237.
- Lo, Chi-Chu; Chao, Pei-Min (1990). "Replacement of carcinogenic solvent HMPA by DMI in insect sex pheromone synthesis". Journal of Chemical Ecology. 16 (12): 3245–3253. doi:10.1007/BF00982095. PMID 24263426.
- McDonald, Chriss E.; Ramsey, Jeremy D.; Sampsell, David G.; Butler, Julie A.; Cecchini, Michael R. (2010). "Tripyrrolidinophosphoric Acid Triamide as an Activator in Samarium Diiodide Reductions". Organic Letters. 12 (22): 5178–5181. doi:10.1021/ol102040s. PMID 20979412.
- Berndt, Mathias; Hölemann, Alexandra; Niermann, André; Bentz, Christoph; Zimmer, Reinhold; Reissig, Hans-Ulrich (2012). "Replacement of HMPA in Samarium Diiodide Promoted Cyclizations and Reactions of Organolithium Compounds". European Journal of Organic Chemistry. 2012 (7): 1299–1302. doi:10.1002/ejoc.201101830. ISSN 1099-0690.
Tripyrrolidinophosphoric acid triamide (TPPA) can replace carcinogenic HMPA as a Lewis basic additive in many reactions involving samarium ketyls. In most cases, yields and selectivities of cyclizations of (het)aryl, alkenyl, and alkynyl ketones are similar.
External links
- "Hexamethylphosphoramide CAS No. 680-31-9" (PDF). Report on Carcinogens (12th ed.). National Toxicology Program, Department of Health and Human Services. 2011.
- "Hexamethyl phosphoramide". NIOSH Pocket Guide to Chemical Hazards. Centers for Disease Control and Prevention, Department of Health and Human Services. 2011.
- Merck Index. 4761 (12th ed.).