Eristalis tenax
Eristalis tenax is a hoverfly, also known as the drone fly, of the order Diptera. Diptera is one of the three largest and most diverse animal groups in the world.[2] Eristalis tenax is common, migratory and cosmopolitan; it is the most widely distributed syrphid species in the world and is known from all regions except the Antarctic. It has been introduced into North America and is widely established. It can be found in gardens and fields all over both Europe[3] and Australia,[4] but have also been found in the Himalayas.[5]
| Eristalis tenax | |
|---|---|
 | |
| Eristalis tenax. Male | |
_female.jpg) | |
| Female | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Subfamily: | |
| Tribe: | |
| Genus: | |
| Subgenus: | Eoseristalis |
| Species: | E. tenax |
| Binomial name | |
| Eristalis tenax | |
| Synonyms | |
Distribution
The larval form of the drone-fly, the rat-tailed maggot, are on every continent except Antarctica and range to the highest latitudes in the North.[6] They aren't prevalent in extremely southern latitudes and in arid areas of Europe, Asia, and Africa.[6] In the United States, they can be found as far north as Alaska and as south as California and Florida.[6]
Description
The appearance of the drone fly can vary drastically.[7] Eristalis tenax is a large, stocky bee mimic. Their eyes are marbled in black. Males have hovering displays. Their average wing length is 9.75–13 mm and their average wingspan is 15 mm. They have a stout appearance, like bees.
The abdomen can vary from dark brown to very orange.[7] Pigmentation also has an important role in the control of body temperature; the black areas down the center of the drone-flies abdomen may absorb solar radiation and so warm the dorsal blood vessel that is right underneath.[7]
Territoriality
The males of E. tenax can be heavily territorial in the summer, guarding territories including flowerbeds or bushes for a chance to mate. The males hover still in the air and dart after intruders to chase them out of the territory.[8]
Researcher observations suggest that male drone flies live in the same territory their entire lives.[6] They mate, feed, and groom in this area defending it against other insects.[6] When male E. tenax of the spring and summer generations stop dispersing, they settle within home ranges that provide them with sheltering, resting, basking, grooming, feeding and mating locations.[9] Away from its "home" a male drone fly rarely responds to other insects.[9] On its mating site, the male is very territorial, attacking alien species like bees, wasps, and butterflies.[9] Being on territorial duty is very demanding, so much so that the males take rest periods when they can outside of their territory.[9] When weather conditions don't allow them to leave their territory, they become increasingly aggressive.[9] Males that live in horizontal territories such as flowerbeds are more likely to notice intruders and thusly are more likely to attack them than males living in vertical territories such as shrubs.[9]
Life history
Egg
The egg is white, covered in a sticky substance, and has an elongated shape.[6]
Larva
The larva is aquatic, and has a cylindrical shape with patches of horizontal folds that divides the body into segments.[6] At each of the segments, two rows of flexible hairs are visible.[6] All drone-fly larvae have a siphon on their posterior end that acts as a respiratory mechanism and also looks like a tail.[6] The siphon can be several times the length of the larva's body.[6]
Pupa
The pupal stage of the drone fly is very similar to the larva stage but they are shorter and thicker.[6] Unlike the larval stage, the pupa have two pairs of cornua (horn-like bumps) on their thorax.[6] The siphon is still present, but is locked in a curved position over the back.[6]
Adult
The adult drone fly is about an inch in length, and can be easily differentiated from honey bees because they don't have a constricted middle between the thorax and the abdomen.[6] They only have two wings while bees have four.[6] There are short brownish- yellow hairs on the thorax and the beginning segment of the abdomen.[6] The adult drone-fly's body is dark brown to black in color, with yellow-orange marks in the side of the second part of the abdomen. Lastly, there is a yellow-orange band that crosses the 3rd abdominal segment.[6]
Biology
Larvae are saprophagous. The larva of E. tenax is a rat-tailed maggot. It lives in drainage ditches, pools around manure piles, sewage, and similar places containing water badly polluted with organic matter.[10] The larvae likely feed on the abundant bacteria living in these places.
When fully grown, the larvae creep out into drier habitats and seek a suitable place to pupate. In doing so, they sometimes enter buildings, especially barns and basements on farms. The pupae are typically 10–12 mm long, grey-brown, oval, and retain the long tail; they look like a tiny mouse.
The adult fly that emerges from the pupa is harmless. It looks somewhat like a drone honey bee, and likely gains some degree of protection from this resemblance to a stinging insect. The adults are called drone flies because of this resemblance. In its natural habitat, E. tenax is more of a curiosity than a problem. Like other hover flies, they are common visitors to flowers,[11] especially in late summer and autumn, and can be significant pollinators. They mainly feed on flowers of carrot and fennel.
Under extremely rare conditions, there have been documented cases of human intestinal myiasis of the rat-tailed maggot (larva of Eristalis tenax). Zumpt proposed a hypothesis called "rectal myiasis". During open defecation in the wilderness, flies attracted to feces may deposit their eggs or larvae near or into the anus, and the larvae then penetrate further into the rectum. They can survive feeding on feces at this site, as long as the breathing tube reaches towards the anus which is quite rare.[10][12]
Myiasis
The drone-fly is reported to have caused accidental myiasis.[6] Myiasis in humans is usually an accident and is caused by the ingestion of contaminated uncooked food or water containing fly larvae.[13] This occurs when fly larvae inhabits a living host by accident, usually from ingestion of contaminated foods. In humans, myiasis can be caused in four ways: intestinal or gastric, nasal, auricular, or anal.[6] Gastric or intestinal is the most common. Most reported myaisis has been reported in countries where nutritional and sanitary conditions are unsatisfactory.[13] There are very few reports of intestinal infestation from India, and Africa.[13] Clinically the symptoms are variable, including cases that are asymptomatic, but usually abdominal pain, nausea and vomiting are some symptoms one with myiasis might face.[6]
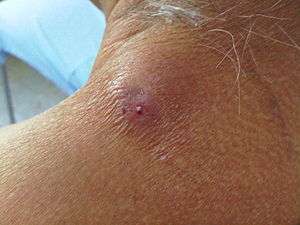
Dronefly larvae are able to survive in the gastric acids due possibly to the fact that they are adapted to living in polluted environments.[6] Myaisis becomes apparent to the host when they notice the larvae in their bowel movements.[6]
Life cycle
Drone-fly eggs are normally laid on surface water and go through three distinct larval stages.[14] The larvae are usually found in still or stagnant water, like water reservoirs or liquid dung.[14] Just before the pupation stage, the larvae leave their aquatic environment.[14]
Life cycle stages
There are still many gaps in the understanding of the drone fly life cycle, and more detailed research is needed.[6]
Eggs
The eggs are deposited near the surface of foul water or decaying organic material.[6] The eggs are laid side by side, perpendicular to the ground. It is still unknown how long it takes for the eggs to hatch.[6]
Larvae
The larvae are aquatic, but there must be enough solid food for the larva to complete development.[6] This is why they are found in water with high levels of organic matter.[6] The siphon on the back of the larvae remains at the surface of the water while the larva moves throughout the water.[6] This allows for the larva to search for food without having to go to the surface to breathe.[6] It was reported that the larvae reproduce by neoteny or paedogenesis, where the larva copies itself. There has only been one observation of this happening.[6]
Pupae
Pupation happens in a drier location than where the larvae develop.[6] It usually happens below the soil surface, where they remain for eight to 10 days.[6] The cornua that appear on the pupa are believed to help respiration because the siphon becomes unusable.[6]
Adults
Females will feed on pollen after they emerge from the pupa so that they are able to get the nutrients they need to complete reproduction.[6] Their next few meals will consist of nectar from daisies, chrysanthemums, and asters.[6]
Toughness
The toughness of E. tenax increases with age; the skin is insoluble even in strong alkaline solutions.[15] There have been reports of decapitated drone-flies living for three days and nights on the stage of a microscope.[15] When it doesn't have an abdomen, an adult drone-fly buzzed for more than an hour.[15] The thorax can even be sliced open for examination, and its muscular bundles inside may twitch when an irritant or needle-point is used.[15] Losing its wings seems to produce very little inconvenience to the fly, when put on a flower it immediately plunged it proboscis into the center and continued to feed for several minutes.[15]
Reproduction

Mating can happen while a pair of E. tenax is flying, with the male uppermost, or on the ground while resting on foliage.[6] After mating, the adult, female lay clusters of about 10 eggs near dirty, contaminated water, sewage, or decomposing organic substances.[6]
Diet
The drone fly's diet consists mostly of honey and the pollen of flowers. During the imago stage, it drinks mostly honey, but will have a little bit of water when it's presented.[15]
Pollen eating
It was found by researchers that when E. tenax traps pollen among the body hairs they are combed off by the front and hind tibia and transferred to pollen retaining bristles on the front and hind tarsi.[16] The pollen retained among the front tarsal bristle are eaten directly from the bristles.[16] Those caught by the hind tarsi are transferred in flight by leg scraping to the front tarsi, where they are then eaten. E. tenax also eats pollen directly from its anther.[16]
Pollination
Diptera are often neglected but are an important group of pollinators, they play a significant role in agricultural biodiversity and the biodiversity of plants everywhere.[2] Hoverflies are considered to be less specialized pollinators than bees and are more effective in open than tubular flowers.[2] There is a significant difference in pollination efficiency between the two taxa.[17]
Mimicry

There is little doubt that the morphological and behavioral similarities between E. tenax and the honey bee are mainly a result of convergent evolution in response to similar food-gathering requirements.[16] Bees are common models for several Dipteran mimics[7] They are similar in their general form, flight, and coloration.[7] There are reports on the genetics of honeybees that show that the factors controlling the coloration are the same as the ones controlling the coloration in E. tenax.[16] In both species branched hairs and spirally grooved bristles act as collectors and retainers of pollen, and leg-scraping that occurs during hovering allows the transfer of pollen to take place, from hind legs to front legs in E. tenax, and in the reverse direction in honey bees.[16]

E. tenax also has partial mimicry of wasps.[7] To improve the wasp mimicry there seems to be an epistatic influence on the Ap gene on the hair color genes.[7] The lightest phenotype of E. tenax is a light yellow, and seen most clearly as yellow bands along its sides, where a wasp has stripes of yellow pigment.[7] The lighter patterns would be less beneficial from wasp mimicry early in the year, before there are a surplus of wasps.[7]
Activity
Drone flies are active during much of the year, from March to December, and sometimes are more numerous than honeybees, especially during autumn in urban areas.[7] Males and females occur in roughly equal numbers in summer and autumn, but males are very rare in the spring when fertilized females come out of hibernation.[7]
See also
References
- Stubbs, Alan E.; Falk, Steven J. (1983). British Hoverflies: An Illustrated Identification Guide. British Entomological & Natural History Society. p. 253, xvpp. ISBN 978-0-9502891-4-4.
- Ssymank, Axel; Kearns, C. A.; Pape, Thomas; Thompson, F. Christian (April 2008). "Pollinating Flies (Diptera): A major contribution to plant diversity and agricultural production". Biodiversity. 9 (1–2): 86–89. doi:10.1080/14888386.2008.9712892. ISSN 1488-8386. S2CID 39619017.
- Francuski, Ljubinka; Djurakic, Marko; Ludoški, Jasmina; Milankov, Vesna (2013-08-01). "Landscape genetics and spatial pattern of phenotypic variation of Eristalis tenax across Europe". Journal of Zoological Systematics and Evolutionary Research. 51 (3): 227–238. doi:10.1111/jzs.12017. ISSN 1439-0469.
- Hull, Frank M. (1937). A Check List of the Syrphidae of Oceania. Honolulu, Hawaii: Bernice P. Bishop Museum.
- Shah1;Jan2;Ahmad Wachkoo3, Ghulam Mustafa1;Ulfat2;Aijaz3 (2014). "A CHECKLIST OF HOVERFLIES (DIPTERA/ SYRPHIDAE) IN THE WESTERN HIMALAYA, INDIA" (PDF). Acta Zoologica Academiae Scientiarum Hungaricae. 60 (4): 283–305.CS1 maint: multiple names: authors list (link)
- "drone fly, rat-tailed maggot - Eristalis tenax (Linnaeus)". entnemdept.ufl.edu. Retrieved 2019-10-31.
- Heal, J. R. (August 1982). "Colour patterns of syrphidae: IV. Mimicry and variation in natural populations of Eristalis tenax". Heredity. 49 (1): 95–109. doi:10.1038/hdy.1982.68. ISSN 1365-2540.
- Fitzpatrick, Sheila M. (1981). Territorial aggression among males of three Syrphid species. The University of British Columbia.
- Wellington, W. G.; Fitzpatrick, Sheila M. (August 1981). "Territoriality in the Drone Fly, Eristalis Tenax (Diptera: Syrphidae)". The Canadian Entomologist. 113 (8): 695–704. doi:10.4039/Ent113695-8. ISSN 1918-3240.
- Aguilera, A; Cid, A; Regueiro, BJ; Prieto, JM; Noya, M (1999). "Intestinal Myiasis Caused by Eristalis tenax". Journal of Clinical Microbiology. 37 (9): 3082. doi:10.1128/JCM.37.9.3082-3082.1999. PMC 85471. PMID 10475752.
- Van Der Kooi, C. J.; Pen, I.; Staal, M.; Stavenga, D. G.; Elzenga, J. T. M. (2015). "Competition for pollinators and intra-communal spectral dissimilarity of flowers". Plant Biology. 18 (1): 56–62. doi:10.1111/plb.12328. PMID 25754608.
- Phillip B. Whish-Wilson (2000). "A possible case of intestinal myiasis due to Eristalis tenax". Medical Journal of Australia. 173 (11): 652. doi:10.5694/j.1326-5377.2000.tb139374.x. Retrieved January 13, 2008.
- Aguilera, A.; Cid, A.; Regueiro, B. J.; Prieto, J. M.; Noya, M. (1999-09-01). "Intestinal Myiasis Caused by Eristalis tenax". Journal of Clinical Microbiology. 37 (9): 3082. doi:10.1128/JCM.37.9.3082-3082.1999. ISSN 0095-1137. PMID 10475752.
- Fischer, O. A.; Mátlová, L.; Dvorská, L.; Švástová, P.; Bartoš, M.; Weston, R. T.; Pavlík, I. (2006-03-01). "Various stages in the life cycle of syrphid flies (Eristalis tenax; diptera: Syrphidae) as potential mechanical vectors of pathogens causing mycobacterial infections in pig herds". Folia Microbiologica. 51 (2): 147–153. doi:10.1007/BF02932171. ISSN 1874-9356. PMID 16821726.
- Bowdler Buckton, George. The Natural History of Eristalis Tenax Or the Drone-fly.
- Holloway, Beverley A. (1976-12-01). "Pollen‐feeding in hover‐flies (Diptera: Syrphidae)". New Zealand Journal of Zoology. 3 (4): 339–350. doi:10.1080/03014223.1976.9517924. ISSN 0301-4223.
- Jauker, Frank; Bondarenko, Birgit; Becker, Heiko C.; Steffan‐Dewenter, Ingolf (2012). "Pollination efficiency of wild bees and hoverflies provided to oilseed rape". Agricultural and Forest Entomology. 14 (1): 81–87. doi:10.1111/j.1461-9563.2011.00541.x. ISSN 1461-9563.
External links
| Wikispecies has information related to Eristalis tenax |
| Wikimedia Commons has media related to Eristalis tenax. |
- External images
- Drone fly on the University of Florida / Institute of Food and Agricultural Sciences Featured Creatures website
