Dihydroorotate dehydrogenase (quinone)
Class 2 dihydroorotate dehydrogenases (DHOQO, EC 1.3.5.2) is an enzyme with systematic name (S)-dihydroorotate:quinone oxidoreductase.[1][2][3][4][5] This enzyme catalyses the electron transfer from dihydroorotate (electron donor) to a quinone (electron acceptor):
- (S)-dihydroorotate + quinone orotate + quinol

| Dihydroorotate:quinone oxidoreductase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.3.5.2 | ||||||||
| CAS number | 59088-23-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
These enzymes differ from class 1 dihydroorotate dehydrogenases (DHODH) on the electron acceptor, on their structure, and on their cellular localization. Since the reaction catalyzed by DHOQOs is both part of the electron transport chain and the pyrimidine de novo synthesis, it has been explored as a possible target for cancer treatment, immunological disorders and bacterial/viral infections. [6][7][8]
Structure
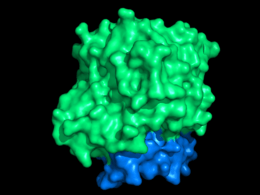
Sructurally, DHOQOs are organized in monomers which adopt a (βα)8 (eightfold beta alpha barrel) fold[9]. The enzyme can be separated in its N-terminal domain (blue in the figure) and in its C-terminal domain (green in the figure).
The N-terminal domain is composed of two amphipathic α-helices (αA – αB) which are responsible for the lipid membrane interaction. This region of the protein is also thought to mediate quinone binding.
Regarding the C-terminal domain, much of its structural elements are shared with the soluble counterparts of DHOQOs. This domain is responsible for the binding of the cofactor FMN (making these enzymes part of the Flavoprotein super-family) and the electron donor dihydroorotate, close to the 8 β-strand core.
There are currently crystallographic structures of DHOQOs from 5 different organisms:
- Escherichia coli (example PDB:1F76)[10]
- Mycobacterium tuberculosis (example PDB: 4XQ6)[11]
- Homo sapiens (example PDB:4IGH)[12]
- Rattus norvegicus (example PDB: 4ORI)[13]
- Plasmodium falciparum (example PDB: 1TV5)[14]
References
- Forman HJ, Kennedy J (November 1978). "Mammalian dihydroorotate dehydrogenase: physical and catalytic properties of the primary enzyme". Archives of Biochemistry and Biophysics. 191 (1): 23–31. doi:10.1016/0003-9861(78)90063-2. PMID 216313.
- Hines V, Keys LD, Johnston M (August 1986). "Purification and properties of the bovine liver mitochondrial dihydroorotate dehydrogenase". The Journal of Biological Chemistry. 261 (24): 11386–92. PMID 3733756.
- Bader B, Knecht W, Fries M, Löffler M (August 1998). "Expression, purification, and characterization of histidine-tagged rat and human flavoenzyme dihydroorotate dehydrogenase". Protein Expression and Purification. 13 (3): 414–22. doi:10.1006/prep.1998.0925. PMID 9693067.
- Fagan RL, Nelson MN, Pagano PM, Palfey BA (December 2006). "Mechanism of flavin reduction in class 2 dihydroorotate dehydrogenases". Biochemistry. 45 (50): 14926–32. doi:10.1021/bi060919g. PMID 17154530.
- Björnberg O, Grüner AC, Roepstorff P, Jensen KF (March 1999). "The activity of Escherichia coli dihydroorotate dehydrogenase is dependent on a conserved loop identified by sequence homology, mutagenesis, and limited proteolysis". Biochemistry. 38 (10): 2899–908. doi:10.1021/bi982352c. PMID 10074342.
- J. Leban, D. Vitt, Human dihydroorotate dehydrogenase inhibitors, a novel approach for the treatment of autoimmune and inflammatory diseases, Arzneimittel-Forschung/Drug Res. (2011). https://doi.org/10.1055/s-0031-1296169
- R.I. Christopherson, S.D. Lyons, P.K. Wilson, Inhibitors of de novo nucleotide biosynthesis as drugs, Acc. Chem. Res. (2002). https://doi.org/10.1021/ar0000509
- M. Löffler, L.D. Fairbanks, E. Zameitat, A.M. Marinaki, H.A. Simmonds, Pyrimidine pathways in health and disease, Trends Mol. Med. (2005). https://doi.org/10.1016/j.molmed.2005.07.003
- D. Lang, R. Thoma, M. Henn-Sax, R. Sterner, M. Wilmanns, Structural evidence for evolution of the β/α barrel scaffold by gene duplication and fusion, Science (80-. ). (2000). https://doi.org/10.1126/science.289.5484.1546
- E. coli Dihydroorotate Dehydrogenase Reveals Structural and Functional Distinction between different classes of dihydroorotate dehydrogenases. DOI: 10.1016/s0969-2126(02)00831-6
- CRYSTAL STRUCTURE OF DIHYDROOROTATE DEHYDROGENSE from MYCOBACTERIUM TUBERCULOSIS. DOI: 10.2210/pdb4XQ6/pdb
- SAR Based Optimization of a 4-Quinoline Carboxylic Acid Analog with Potent Anti-Viral Activity. DOI: 10.1021/ml300464h
- Fluorine Modulates Species Selectivity in the Triazolopyrimidine Class of Plasmodium falciparum Dihydroorotate Dehydrogenase Inhibitors. DOI: 10.1021/jm500481t
- Structure of Plasmodium falciparum dihydroorotate dehydrogenase with a bound inhibitor. DOI: 10.1107/S0907444905042642
External links
- Dihydroorotate+dehydrogenase+(quinone) at the US National Library of Medicine Medical Subject Headings (MeSH)