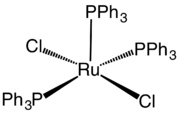
Dichlorotris(triphenylphosphine)ruthenium(II)
Dichlorotris(triphenylphosphine)ruthenium(II) is a coordination complex of ruthenium. It is a chocolate brown solid that is soluble in organic solvents such as benzene. The compound is used as a precursor to other complexes including those used in homogeneous catalysis.
 | |
| Names | |
|---|---|
| IUPAC name
Dichlorotris(triphenylphosphine)ruthenium(II) | |
| Other names
Ruthenium tris(triphenylphosphine) dichloride; Tris(triphenylphosphine)dichlororuthenium; Tris(triphenylphosphine)ruthenium dichloride;Tris(triphenylphosphine)ruthenium(II) dichloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.035.957 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C54H45Cl2P3Ru | |
| Molar mass | 958.83 g/mol |
| Appearance | Black Crystals or Red-Brown |
| Density | 1.43 g cm−3 |
| Melting point | 133 °C; 271 °F; 406 K |
| Structure | |
| Monoclinic | |
| C2h5-P21/c | |
a = 18.01 Å, b = 20.22 Å, c = 12.36 Å α = 90°, β = 90.5°, γ = 90° | |
| Octahedral | |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H302, H312, H332 |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and basic properties
RuCl2(PPh3)3 is the product of the reaction of ruthenium trichloride trihydrate with a methanolic solution of triphenylphosphine.[1][2]
- 2 RuCl3(H2O)3 + 7 PPh3 → 2 RuCl2(PPh3)3 + 2 HCl + 5 H2O + 1 OPPh3
The coordination sphere of RuCl2(PPh3)3 can be viewed as either five-coordinate or octahedral. One coordination site is occupied by one of the hydrogen atoms of a phenyl group.[3] This Ru---H an agostic interaction is long (2.59 Å) and weak. The low symmetry of the compound is reflected by the differing lengths of the Ru-P bonds: 2.374, 2.412, and 2.230 Å.[4] The Ru-Cl bond lengths are both 2.387 Å.
Reactions
In the presence of excess of triphenylphosphine, RuCl2(PPh3)3 binds a fourth phosphine to give black RuCl2(PPh3)4. The triphenylphosphine ligands in both the tris(phosphine) and tetrakis(phosphine) complexes are labile and are readily substituted by other ligands. Notably, the tetrakis(phosphine) complex is a precursor to the Grubbs catalysts.[5]
Dichlorotris(triphenylphosphine)ruthenium(II) reacts with carbon monoxide to produce the all trans isomer of dichloro(dicarbonyl)bis(triphenylphosphine)ruthenium(II).
- RuCl2(PPh3)3 + 2 CO → trans,trans,trans-RuCl2(CO)2(PPh3)2 + PPh3
This kinetic product isomerizes to the cis adduct during recrystallization. trans-RuCl2(dppe)2 forms upon treating RuCl2(PPh3)3 with dppe.
- RuCl2(PPh3)3 + 2 dppe → RuCl2(dppe)2 + 3 PPh3
RuCl2(PPh3)3 catalyzes the decomposition of formic acid into carbon dioxide and hydrogen gas in the presence of an amine.[6] Since carbon dioxide can be trapped and hydrogenated on an industrial scale, formic acid represents a potential storage and transportation medium.
Use in organic synthesis
RuCl2(PPh3)3 facilitates oxidations, reductions, cross-couplings, cyclizations, and isomerization. It is used in the Kharasch addition of chlorocarbons to alkenes.[7]
ruthenium(II).png)
Dichlorotris(triphenylphosphine)ruthenium(II) serves as a precatalyst for the hydrogenation of alkenes, nitro compounds, ketones, carboxylic acids, and imines. On the other hand, it catalyzes the oxidation of alkanes to tertiary alcohols, amides to t-butyldioxyamides, and tertiary amines to α-(t-butyldioxyamides) using tert-butyl hydroperoxide. Using other peroxides, oxygen, and acetone, the catalyst can oxidize alcohols to aldehydes or ketones. Using dichlorotris(triphenylphosphine)ruthenium(II) the N-alkylation of amines with alcohols is also possible (see "borrowing hydrogen").[7]

RuCl2(PPh3)3 efficiently catalyzes carbon-carbon bond formation from cross couplings of alcohols through C-H activation of sp3 carbons in the presence of a Lewis acid.[8]

References
- Stephenson, T. A.; Wilkinson, G. "New Complexes of Ruthenium (II) and (III) with Triphenylphosphine, Triphenylarsine, Trichlorostannate, Pyridine, and other Ligands", J. Inorg. Nucl. Chem., 1966, 28, 945-956. doi:10.1016/0022-1902(66)80191-4
- P. S. Hallman, T. A. Stephenson, G. Wilkinson "Tetrakis(Triphenylphosphine)Dichloro-Ruthenium(II) and Tris(Triphenylphosphine)-Dichlororuthenium(II)" Inorganic Syntheses, 1970 Volume 12, . doi:10.1002/9780470132432.ch40
- Sabo-Etienne, S.; Gellier, M., "Ruthenium: Inorganic and Coordination Chemistry", Encyclopedia of Inorganic Chemistry, 2006, John Wiley & Sons. doi:10.1002/0470862106.ia208
- La Placa, S. J.; Ibers, J.A., "A Five-Coordinated d6 Complex: Structure of Dichlorotris(triphenylphosphine)ruthenium(II)", Inorganic Chemistry, 1965, 4, 778-78. doi:10.1021/ic50028a002
- Georgios C. Vougioukalakis, Robert H. Grubbs "Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts" Chem. Rev., 2010, volume 110, pp 1746–1787. doi:10.1021/cr9002424
- Loges, B.; Boddien, A.; Junge, H.; Beller, M., "Controlled Generation of Hydrogen from Formic Acid Amine Adducs at Room Temperature and Application in H2/O2 Fuel Cells", Angew. Chem. Int. Ed., 2008, 47, 3962-3965. doi: 10.1002/anie.200705972
- Plummer, J. S.; Shun-Ichi, M.; Changjia, Z. "Dichlorotris(triphenylphosphine)ruthenium(II)", e-EROS Encyclopedia of Reagents for Organic Synthesis, 2010, John Wiley. doi:10.1002/047084289X.rd137.pub2
- Shu-Yu, Z.; Yong-Qiang, T.; Chun-An, F.; Yi-Jun, J.; Lei, S.; Ke, C.; En, Z.; "Cross-Coupling Reactions between alcohols through sp3 C-H Activation Catalyzed by a Ruthenium/Lewis Acid System" Chem. Eur. J., 2008, 14, 10201-10205. doi:10.1002/chem.200801317