Tetrolic acid
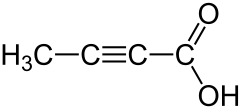
Tetrolic acid (2-butynoic acid) is a short-chain unsaturated carboxylic acid, described by the formula CH3-C≡C-CO2H. Salts and esters of tetrolic acid are known as tetrolates.
 | |
_crystals.jpg) | |
| Names | |
|---|---|
| Preferred IUPAC name
But-2-ynoic acid | |
| Other names
2-Butynoic acid But-2-ynoic acid Butynoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.815 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H4O2 | |
| Molar mass | 84.074 g·mol−1 |
| Density | 0.9641 g/cm3[1] |
| Melting point | 78 °C (172 °F; 351 K)[1] |
| Boiling point | 203 °C (397 °F; 476 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
The first reported synthesis[2] of tetrolic acid is believed to be by German chemist Johann Georg Anton Geuther in 1871 as part of his work investigating the derivatives of ethyl acetoacetate.
Production
Tetrolic acid is manufactured[3] on a commercial scale by treatment of propyne with a strong base (to form an acetylide), followed by carbon dioxide:
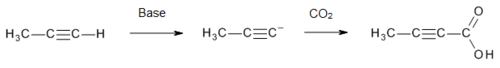
Properties
Tetrolic acid is highly soluble in polar solvents (water, ethanol) and may be recrystallized from non polar solvents (such as heptane, toluene). The compound is a white crystalline solid which can exist in two polymorphous crystalline forms.[6]
The proton nuclear magnetic resonance (1H-NMR) spectrum in deuterated dimethyl sulfoxide shows a characteristic singlet peak at 1.99 ppm corresponding to the -CH3 protons.
Tetrolic acid sublimes at temperatures above 20°C, and should ideally be stored in a sealed container in a refrigerator.[7]
Safety
Tetrolic acid is thermally unstable at high temperatures. Testing using accelerated rate calorimetry (ARC) showed exothermic onset from 135 °C, precluding short-path distillation as a means of purification.[7]
References
| Wikimedia Commons has media related to Tetrolic acid. |
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 3.88. ISBN 1439855110.
- Geuther, A. (1871). "Ethyldiacetic Acid and some of its Derivatives". J. Chem. Soc. 24: 812–837. doi:10.1039/JS8712400808.
- Smith, W. (1973) "Preparation of tetrolic acid" U.S. Patent 3,752,848A
- Hartzoulakis, Basil; Gani, David (1994). "Synthesis of (2S, 3R)- and (2S, 3S)-3-methylglutamic acid". J. Chem. Soc., Perkin Trans. 1. 1994 (18): 2525–2531. doi:10.1039/P19940002525.
- Kauer, J. C.; Brown, M. (1962). "Tetrolic Acid (2-Butynoic Acid)". Organic Syntheses. 42: 97. doi:10.15227/orgsyn.042.0097.; Collective Volume, 5, p. 1043
- Flakus, Henryk T.; Hachuła, Barbara (2008). "Effects of "excessive" exciton interactions in polarized IR spectra of the hydrogen bond in 2-butynoic acid crystals: Proton transfer induced by dynamical co-operative interactions involving hydrogen bonds". Chemical Physics. 345 (1): 49–64. Bibcode:2008CP....345...49F. doi:10.1016/j.chemphys.2008.01.035.
- Golden, M. (2019). "Thermal Stability of 2-Butynoic Acid (Tetrolic acid)". Org. Process Res. Dev. 23, (5) (5): 1101–1104. doi:10.1021/acs.oprd.9b00106.