Malonic acid
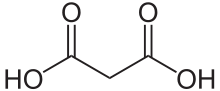
Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (malon) meaning 'apple'.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Malonic acid[1] | |
| Preferred IUPAC name
Propanedioic acid | |
| Other names
Malonic acid Methanedicarboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.003 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H4O4 | |
| Molar mass | 104.061 g·mol−1 |
| Density | 1.619 g/cm3 |
| Melting point | 135 to 137 °C (275 to 279 °F; 408 to 410 K) (decomposes) |
| Boiling point | decomposes |
| 763 g/L | |
| Acidity (pKa) | pKa1 = 2.83[2] pKa2 = 5.69[2] |
| -46.3·10−6 cm3/mol | |
| Related compounds | |
Other anions |
malonate |
Related carboxylic acids |
acetic acid oxalic acid propionic acid tartronic acid acrylic acid butyric acid succinic acid fumaric acid |
Related compounds |
propanone propionaldehyde propanedial dimethyl malonate |
| Hazards | |
| Safety data sheet | External MSDS RESOURCE NO LONGER MAINTAINED |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biochemistry
Malonic acid is often mistakenly believed to occur in beetroot at high concentration. However, a study on the composition of sugar beet liquors revealed no malonic acid.[3] It exists in its normal state as white crystals. Malonic acid is the classic example of a competitive inhibitor: It acts against succinate dehydrogenase[4] (complex II) in the respiratory electron transport chain.
Preparation
A classical preparation of malonic acid starts from chloroacetic acid:[5]

Sodium carbonate generates the sodium salt, which is then reacted with sodium cyanide to provide the cyano acetic acid salt via a nucleophilic substitution. The nitrile group can be hydrolyzed with sodium hydroxide to sodium malonate, and acidification affords malonic acid. Industrially, however, malonic acid is produced by hydrolysis of dimethyl malonate or diethyl malonate.[6]
Malonic acid was first prepared in 1858 by the French chemist Victor Dessaignes (1800-1885) via the oxidation of malic acid.[7]
Malonic acid has been produced through fermentation of glucose.[8]
Organic reactions
In a well-known reaction, malonic acid condenses with urea to form barbituric acid. Malonic acid is also frequently used as an enolate in Knoevenagel condensations or condensed with acetone to form Meldrum's acid. The esters of malonic acid are also used as a −CH2COOH synthon in the malonic ester synthesis.
Additionally, the coenzyme A derivative of malonate, malonyl-CoA, is an important precursor in fatty acid biosynthesis along with acetyl CoA. Malonyl CoA is formed from acetyl CoA by the action of acetyl-CoA carboxylase, and the malonate is transferred to an acyl carrier protein to be added to a fatty acid chain.
Condensed phase thermochemistry data
| Quantity | Value (Units) | Method | Comment |
|---|---|---|---|
| ΔfH°solid | −891.1±0.4 kJ/mol | Ccb | ALS[9] |
| ΔcH°solid | −861.15±0.63 kJ/mol | Ccb | Corresponding ΔfHºsolid = −891.02 kJ/mol (simple calculation by NIST; no Washburn corrections); ALS[9] |
| ΔcH°solid | −861.57±0.63 kJ/mol | Ccb | Corresponding ΔfHºsolid = −890.61 kJ/mol (simple calculation by NIST; no Washburn corrections); ALS[10] |
| S°solid, 1 bar | 149.00 J/mol⋅K | N/A | crystalline, II phase; DH[11] |
Constant pressure heat capacity of solid:
| Cp, solid (J/mol⋅K) | Temperature (K) | Comment |
|---|---|---|
| 127.63 | 298.15 | crystalline, II phase; T = 10 to 370 K.; DH[11] |
Malonic acid in fruits
Malonic acid is a naturally occurring substance found in some fruits. In citrus, fruits produced in organic farming contain higher levels of malonic acid than fruits produced in conventional agriculture.[12]
Applications
Malonic acid is a precursor to specialty polyesters. It can be converted into 1,3-propanediol for use in polyesters and polymers and a projected market size of $621.2 million by 2021. It can also be a component in alkyd resins, which are used in a number of coatings applications for protecting against damage caused by UV light, oxidation, and corrosion. One application of malonic acid is in the coatings industry as a crosslinker for low-temperature cure powder coatings, which are becoming increasingly valuable for heat sensitive substrates and a desire to speed up the coatings process.[13] The global coatings market for automobiles was estimated to be $18.59 billion in 2014 with projected combined annual growth rate of 5.1% through 2022.[14]
It is used in a number of manufacturing processes as a high value specialty chemical including the electronics industry, flavors and fragrances industry, specialty solvents, polymer crosslinking, and pharmaceutical industry. In 2004, annual global production of malonic acid and related diesters was over 20,000 metric tons.[15] Potential growth of these markets could result from advances in industrial biotechnology that seeks to displace petroleum-based chemicals in industrial applications.
Malonic acid was listed as one of the top 30 chemicals to be produced from biomass by the US Department of Energy.[16]
In food and drug applications, malonic acid can be used to control acidity, either as an excipient in pharmaceutical formulation or natural preservative additive for foods.
Malonic acid is used as a building block chemical to produce numerous valuable compounds,[17] including the flavor and fragrance compounds gamma-nonalactone, cinnamic acid, and the pharmaceutical compound valproate.
Malonic acid (up to 37.5% w/w) has been used to cross-link corn and potato starches to produce a biodegradable thermoplastic; the process is performed in water using non-toxic catalysts.[18][19] Starch-based polymers comprised 38% of the global biodegradable polymers market in 2014 with food packaging, foam packaging, and compost bags as the largest end-use segments.[20]
Eastman Kodak company and others use malonic acid and derivatives as a surgical adhesive.[21]
References
- ChemSpider lists the name 'malonic acid' as a valid, expert-verified IUPAC name.
- pKa Data Compiled by R. Williams (pdf; 77 kB) Archived 2010-06-02 at the Wayback Machine
- Stark, J.B. (1950). "Composition of Sugar Beet Liquors". Industrial and Engineering Chemistry. 43 (3): 603–605. doi:10.1021/ie50495a018.
- Pardee, Arthur B.; Potter, Van R. (26 October 1948). "Malonate Inhibition of Oxidations in the Krebs Tricarboxylic Acid Cycle" (PDF). Journal of Biological Chemistry (178): 241–250. Retrieved 5 June 2015.
- Nathan Weiner. "Malonic acid". Organic Syntheses.; Collective Volume, 2, p. 376
- Production of malonic acid, retrieved 2015-12-11
- See:
- Dessaignes (1858) "Note sur un acide obtenu par l'oxydation de l'acide malique" (Note on an acid obtained by oxidation of malic acid), Comptes rendus, 47 : 76-79.
- Dessaignes (1858) "Ueber eine durch Oxydation der Aepfelsäure erhaltene Säure" (On an acid obtained by oxidation of malic acid) Annalen der Chemie und Pharmacie, 107 : 251-254.
- Recombinant host cells for the production of malonate. PCT/US2013/029441 2012.
- Wilhoit, R.C.; Shiao, D., Thermochemistry of biologically important compounds. Heats of combustion of solid organic acids., J. Chem. Eng. Data, 1964, 9, 595-599.
- Verkade, P.E.; Hartman, H.; Coops, J., Calorimetric researches. X. Heats of combustion of successive terms of homologous series: dicarboxylic acids of the oxalic acid series, Rec. Trav. Chim. Pays/Bas, 1926, 45, 373-393.
- Fukai, M.; Matsuo, T.; Suga, H., Thermodynamic properties of phase transitions in malonic acid and its deuterated analog, Thermochim. Acta, 1991, 183, 215-243.
- Duarte, A.M.; Caixeirinho, D.; Miguel, M.G.; Sustelo, V.; Nunes, C.; Fernandes, M.M.; Marreiros, A. (2012). "Organic Acids Concentration in Citrus Juice from Conventional Versus Organic Farming". Acta Horticulturae. 933 (933): 601–606. doi:10.17660/actahortic.2012.933.78. hdl:10400.1/2790. ISSN 0567-7572.
- Fäcke, T., Subramanian R., Dvorchak, M., Feng, S. Diethylmalonate Blocked Isocyanates As Crosslinkers For Low Temperature Cure Powder Coatings. 2004 International Waterborne, High-Solids, and Powder Coatings Symposium.
- James, S. Global Automotive Coatings Market. 2015 Grand View Research Market Report
- "Malonic acid diesters" (PDF). Inchem. UNEP Publications.
- Werpy, T., Petersen, G. Top Value Added Chemicals from Biomass. 2004 US Department of Energy.
- Hildbrand, S.; Pollak, P. Malonic Acid & Derivatives. March 15, 2001. Ullmann's Encyclopedia of Industrial Chemistry
- Netravali, A., Dastidar, T. Crosslinked native and waxy starch resin compositions and processes for their manufacture. 2013 US. Appl No. 14/418,940.
- Dastidar TG, Netravali AN, "'Green' crosslinking of native starches with malonic acid and their properties." Carbohydrate Polymers, 90:1620-1628 (2012)
- "Biodegradable Polymers," Chemical Economics Handbook (May 2015)
- Hawkins, G., Fassett, D. Surgical Adhesive Compositions. 1971 US Patent No. 3,591,676.