Corticovirus
Corticovirus is a genus of viruses in the family Corticoviridae.[2] Corticoviruses are bacteriophages; that is, their natural hosts are bacteria. The genus contains two species, including the type species Pseudoalteromonas virus PM2 (also known as Pseudoalteromonas phage PM2 or bacteriophage PM2).[2][3] The name is derived from Latin cortex, corticis (meaning 'crust' or 'bark'). However, prophages closely related to PM2 are abundant in the genomes of aquatic bacteria, suggesting that the ecological importance of corticoviruses might be underestimated.[4] Bacteriophage PM2 was first described in 1968 after isolation from seawater sampled from the coast of Chile.[5]
| Corticovirus | |
|---|---|
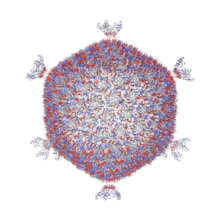 | |
| The assembled capsid made of P2 and P3 proteins.[1] | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Varidnaviria |
| Kingdom: | Bamfordvirae |
| Phylum: | Preplasmiviricota |
| Order: | Vinavirales |
| Family: | Corticoviridae |
| Genus: | Corticovirus |
| Type species | |
| Pseudoalteromonas virus PM2 | |
Taxonomy
Group: dsDNA
- Family: Corticoviridae
- Genus: Corticovirus
- Pseudoalteromonas virus Cr39582
- Pseudoalteromonas virus PM2
Virology
The virons consist of a round, icosahedral, non-enveloped capsid of a diameter of 60 nm and an internal lipid membrane located between outer and inner protein shell.[6] The shells are composed of three layers whose surfaces reveals a pattern with distinctive features,[7] including bush-like spikes protruding from the twelve vertices.[8]
The icosahedral capsid (T = 21) is 56 nanometers (nm) in diameter and is composed of 1200 P1 (spike) and 60 P2 (capsid) proteins. The pentameric receptor-binding spikes protrude from the 12 fivefold axes. The capsid encloses an internal lipid core containing the structural proteins P3 to P10.
Genome
The genome is not segmented, constitutes 13% of the virus's weight and contains a single molecule of circular, supercoiled, double-stranded DNA of 10 kilobases in length. The genome has a g + c content of 43%.[9] It encodes ~21 proteins.
Transcription is organised into three operons.
Replication of the genome is via a rolling-circle mechanism, initiated by the virus encoded endonuclease P12.
| Genus | Structure | Symmetry | Capsid | Genomic arrangement | Genomic segmentation |
|---|---|---|---|---|---|
| Corticovirus | Polyhedral | T=21 | Non-enveloped | Circular | Monopartite |
Life cycle
Viral replication is cytoplasmic. Entry into the host cell is achieved by adsorption to the host cell surface followed by fusion of the viral membrane with the outer membrane of the host cell and subsequent genome delivery into the cell interior.[10] DNA-templated transcription is the method of transcription. Bacteria of the genus Pseudoalteromonas serve as the natural host. Corticovirus PM2 is a lytic virus and at the end of the infection cycle disrupts the host cell using a unique lysis system consisting of phage-encoded proteins P17 and P18 as well as an unidentified host autolysin.[11] However, identification of PM2-like proviruses in bacterial genomes indicates that other members of this family might be temperate viruses. Transmission routes are passive diffusion.[3]
| Genus | Host details | Tissue tropism | Entry details | Release details | Replication site | Assembly site | Transmission |
|---|---|---|---|---|---|---|---|
| Corticovirus | Bacteria | None | Injection | Lysis | Cytoplasm | Cytoplasm | Passive diffusion |
References
- Abrescia, Nicola G.A.; Grimes, Jonathan M.; Kivelä, Hanna M.; Assenberg, Rene; Sutton, Geoff C.; Butcher, Sarah J.; Bamford, Jaana K.H.; Bamford, Dennis H.; Stuart, David I. (September 2008). "Insights into Virus Evolution and Membrane Biogenesis from the Structure of the Marine Lipid-Containing Bacteriophage PM2". Molecular Cell. 31 (5): 749–761. doi:10.1016/j.molcel.2008.06.026. PMID 18775333.
- "Corticoviridae". ICTV Online (10th) Report.
- "Viral Zone". ExPASy. Retrieved 15 June 2015.
- Krupovic M, Bamford DH (2007). "Putative prophages related to lytic tailless marine dsDNA phage PM2 are widespread in the genomes of aquatic bacteria" (PDF). BMC Genomics. 8: 236. doi:10.1186/1471-2164-8-236. PMC 1950889. PMID 17634101.
- Espejo, Romilio T.; Canelo, Eliana S. (April 1968). "Properties of bacteriophage PM2: A lipid-containing bacterial virus". Virology. 34 (4): 738–747. doi:10.1016/0042-6822(68)90094-9.
- Kiveld, H.M., Kalkkinen, N. and Bamford, D.H. (2002). Bacteriophage PM2 has a protein capsid surrounding a spherical lipid-protein core" J. Virol. 76, 8169-8178.
- Kiveld, H.M., Männistö, R.H., Kalkkinen, N. and Bamford, D.H. (1999). Purification and protein composition of PM2, the first lipid-containing bacterial virus to be isolated" Virology 262, 364-374.
- Harrison, S.C., Caspar, D.L., Camerini-Otero, R.D. and Franklin, R.M. (1971). Lipid and protein arrangement in bacteriophage PM2. Nat. New Biol., 229, 197-201.
- Männistö, R.H., Kivelä, H.M., Paulin, L., Bamford, D.H. and Bamford, J.K.H. (1999). The complete genome sequence of PM2, the first lipid-containing bacterial virus to be isolated" Virology 262, 355-363.
- Cvirkaite-Krupovic V, Krupovic M, Daugelavicius R, Bamford DH (2010). "Calcium ion-dependent entry of the membrane-containing bacteriophage PM2 into its Pseudoalteromonas host". Virology. 405 (1): 120–128. doi:10.1016/j.virol.2010.05.021. PMID 20646729.
- Krupovic M, Daugelavicius R, Bamford DH (2007). "A novel lysis system in PM2, a lipid-containing marine double-stranded DNA bacteriophage". Mol Microbiol. 64 (6): 1635–48. doi:10.1111/j.1365-2958.2007.05769.x. PMID 17555443.