Common house gecko
The common house gecko (Hemidactylus frenatus) (not to be confused with the Mediterranean species Hemidactylus turcicus known as Mediterranean house gecko), is a gecko native of Southeast Asia. It is also known as the Pacific house gecko, the Asian house gecko, wall gecko, house lizard, or moon lizard.
| Common house gecko | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Family: | Gekkonidae |
| Genus: | Hemidactylus |
| Species: | H. frenatus |
| Binomial name | |
| Hemidactylus frenatus | |
 | |
Most geckos are nocturnal, hiding during the day and foraging for insects at night. They can be seen climbing walls of houses and other buildings in search of insects attracted to porch lights and is immediately recognisable by its characteristic chirp.
They grow to a length of between 75–150 mm (3–6 in), and live for about 5 years. These small geckos are non-venomous and not harmful to humans. Most medium to large geckos are docile, but may bite if distressed, which can pierce skin. A tropical gecko, Hemidactylus frenatus thrives in warm, humid areas where it can crawl around on rotting wood in search of the insects it eats, as well as within urban landscapes. The animal is very adaptable and may prey on insects and spiders, displacing other gecko species which are less robust or behaviourally aggressive.
Habitat and diet


The common house gecko is by no means a misnomer, displaying a clear preference for urban environments. The synanthropic gecko displays a tendency to hunt for insects in close proximity to urban lights.[3] They have been found in bushland, but the current evidence seems to suggest they have a preference for urban environments, with their distribution being mostly defined by areas within or in close proximity to city bounds.[4]
The common house gecko appears to prefer areas in the light which are proximal to cracks, or places to escape. Geckos without an immediate opportunity to escape potential danger display behavioural modifications to compensate for this fact, emerging later in the night and retreating earlier in the morning.[5] Without access to the urban landscape, they appear to prefer habitat which is composed of comparatively dense forest or eucalypt woodland which is proximal to closed forest.[6]
The selection of primarily urban habitats makes available the preferred foods of the common house gecko. The bulk of the diet of the gecko is made up of invertebrates, primarily hunted around urban structures.[3] Primary invertebrate food sources include cockroaches, termites, some bees and wasps, butterflies, moths, flies, spiders, and several beetle groupings.[3] There is limited evidence that cannibalism can occur in laboratory conditions, but this is yet to be observed in the wild.[7]
Distribution
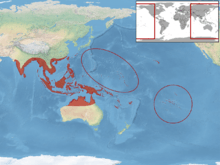
The common house gecko is prolific through the tropics and subtropics. It is capable of existing in an ecologically analogous place with other Hemidactylus species.[8] Despite being native throughout South East Asia, recent introductions, both deliberate and accidental have seen them recorded in the Deep South of the United States, large parts of tropical and sub-tropical Australia, and many other countries in South and Central America, Africa, South Asia, and the Middle East. Their capacity to withstand a wide range of latitudes is also partially facilitated by their capacity to enter a state of brumation during colder months. The prospect of increased climate change interacts synergistically with increased urbanisation, greatly increasing the prospective distribution of the common house gecko.[8] Due to concerns over its potential capacity as an invasive species, there are efforts to limit their introduction and presence in locations where they could be a risk to native gecko species.
In Mexico, H. frenatus was first collected in Acapulco, Guerrero, in March of 1895 and found to be well established there and in the surrounding regions by the early 1940s. It was likely introduced through shipping and cargo. H. frenatus now occurs throughout the lowlands of Mexico on both the Atlantic and Pacific versants including the Yucatan Peninsula, and Baja California, with records from 21 of the 32 Mexican states. Most records of H. frenatus in Mexico are from buildings such as homes, hotels, and other structure in cities and towns, with only a few reports of the species in natural habitat, and its impact, if any, on native fauna there is unknown. [9]
As an invasive species
There is evidence to suggest that the presence of Hemidactylus frenatus has negatively impacted native gecko populations throughout tropical Asia, Central America and the Pacific.[8]
Some species which have been displaced include:
- Lepidodactylus lugubris[10][11][12][13]
- Hemidactylus Garnotti[10][11][12][13]
- The Nactus Genus on the Mascarene Islands (3 are now considered to be extinct)[14]
As an introduced species, they pose a threat through the potential introduction of new parasites and diseases but have potential negative impacts which extend beyond this.[15] The primary cause for concern appears to exist around their exclusionary behaviour and out-competition of other gecko species.[7][11] Mechanistically, three explanations have been derived to justify the capacity of H. frenatus to outcompete other Gecko species:
- Possessing a larger body size. They fail to displace larger native species, such as the Robust Velvet Gecko.[16]
- Male H. frenatus displaying higher levels of aggression than females of other gecko species (particularly parthenogenic species with asexual females).
- Sexual females displaying an increased capacity to compete in comparison to Asexual females.[11]
These differences provide H. frenatus a competitive edge in the limited urban areas they preferentially inhabit, particularly those with high degrees of habitat fragmentation.[10] To compound this, they also are capable of operating on higher densities, which leads to an increase in Gekkonidae sightings and biomass in an area, even after reducing native species density.[10] The common house gecko also displays a higher tolerance to high light levels, which may allow for an increased risk-reward pay off in hunting endeavours. There is also some limited evidence for cannibalism, hunting on other small Gecko species, particularly juveniles.[7] Most of this evidence is in laboratory conditions, with several studies failing to find evidence of cannibalism in the wild for this species.[10][17]
Some males are more territorial than others. Territorial males will display larger heads, with a more pronounced head shape. This increase in head size incurs the cost of a poorer performance in escape sprint time. This suggests selective pressure prioritises the biting force capacity of the male, over their capacity to escape quickly. On the contrary, increases in female head size are met with a proportionate increase in hind limb length and no decrease in speed. Though both sexes use escape sprinting as a survival strategy, males are more likely to need to stop and fight using biting, due to the reduced mobility caused by disproportionate head to hind leg size, which in turn is correlated with localised territorial behaviours.[18]
The success of the common house gecko can also be explained through other elements of competition, such as postural displays and movement patterns. An example of this is how the common house gecko can trigger an "avoidance response" in the Mourning Gecko, causing it to avoid a specific area where food may become available.[11] Though triggering avoidance in other species, they themselves can tolerate the presence of other gecko species well, regardless of whether those species are smaller or larger, faster or slower, or more physically aggressive or not.[8][10][14] This allows them greater access to feeding areas and territories, making them a highly successful invasive species.
Physiology
The common house gecko is ectothermic (“cold blooded”) and displays a variety of means of thermoregulating through behaviour. Its physiology has ramifications for its distribution and nature of interaction with native species, as well as reproductive success as an introduced species.
Metabolically, the demand of the common house gecko is not significantly variable from other lizard species of a similar size, with Oxygen consumption appearing congruent with trends observed in other tropical, subtropical and temperate species of gecko. Thermal independence exists between 26-35 degrees, with some capacity to self regulate temperature. This means that where the environmental temperature is 26-35 degrees, the common house gecko can modify body temperature through behavioural adaptations. Breathing rates of the Gecko are temperature dependent above this maximal heat, but independent as it grows colder.[19] There are behavioural mechanisms of thermoregulation present, such as the selection of sunlight[20] and substrates on which they sit.
The common house gecko can be best defined as quinodiurnal. This means they thermoregulate during the daytime and forage at night.[21] An active form of this thermoregulation includes the presence of the Gecko in lighter environments, proximal to cracks in the substrate. As such, there is a close relationship between activity levels and correlated air temperature.[5] Timing of the circadian rhythm of the common house gecko is further impacted by light levels. This rhythm tends to involve the highest population presence around midnight, with highest activity levels just after sunset,[22] with a gradual reduction until dawn. Daily cycle differences from place to place can generally be explained by environmental factors such as human interaction, and structural features.[5] A peak in hunting activity after dark places them in an ideal spot to take advantage of invertebrate congregation around artificial lighting in the urban environment.
Due to this level of dependence on the environment, drops in temperature may act as a leading indicator for reduced Gecko sightings in the medium term. Acute weather events such as rain or wind will result in acute decreases in Gecko sightings within that environment. It is unsure what impact these phenomena may have on the long term on distribution and the capacity of the common house gecko to compete with other gecko species.
There is some weak evidence to suggest a trend towards higher temperature for females, which has an evolutionary advantage of increasing the speed of egg development. However, there is no statistically significant data to support this.[21]
Due to them being a species which is adapted for tropical or subtropical environments, there appear to be few physiological adaptations designed to prevent water loss. This may limit their capacity to thrive in arid or semi-arid environments.[19]
Reproductive biology
H. frenatus has a similar gonad structure to the remainder of the gekkonid family. It is possible to differentiate the sex of larger common house geckos, with individuals which are larger than 40mm typically displaying differentiated gonads. Differentiated gonads are most clearly seen with a swelling at the entrance to the cloaca caused by the copulatory organs in males. Females lay a maximum of 2 hard-shelled eggs at any single time, with each descending from a single oviduct. Up to 4 eggs can exist within the ovaries in differing stages of development. This shortens the potential turn around between egg-laying events in gravid females.[23] Females produce a single egg per ovary per cycle. This means they are considered monoautochronic ovulatory.[24]
Within the testes, mature sperm are found in the Geckos year-round and are able to be stored within the oviduct of the female. Sperm can be stored for a period of time as long as 36 weeks. This provides a significantly increased chance of colonisation of new habitats, requiring smaller populations to be transplanted for a chance of success. However, longer storage time of sperm within the female is associated with negative survival outcomes and hatching, possibly due to sperm age. Sperm is specifically stored between the uterine and infundibular components of the oviduct. The capacity to store sperm enables a degree of asynchrony between ovulation, copulation and laying of eggs.[24] The capacity to store sperm is useful in island colonisation events, providing females which may be isolated the capacity to reproduce even if they have been separated from a male for some time.[25] In laboratories, one mating event may produce as many as 7 viable egg clutches. This eliminates the need for parthenogenesis and allows the young to include both male and female offspring, with one mating event leading to multiple clutches of eggs being laid. This reduced need for asexual reproduction increases the fitness of young through hybrid vigour and increased diversity.[24] As well as this, sexually reproducing geckos are reported to be more robust and have higher survival rates than those which reproduce asexually.[8]
There is a positive correlation between size and viability of eggs, with larger Geckos having eggs which were more likely to survive. There is also a correlation between warmer year-round temperatures and consistent food supply with reproductive seasonality, with Geckos with constant food and temperatures being less likely to develop fat deposits on their stomach, and more likely to be constantly reproductive.[23]
Genetics
Two distinct karyotypes of the common house gecko appear to exist, one with 40 chromosomes and one with 46 chromosomes.[26][27] This could be explained through an intraspecific variation of karyotype, or the possibility of two distinct species being misidentified. Morphological analysis seems particularly congruent with the suggestion that they indeed are different species.[27][28] Taxonomic revision may be required as a greater understanding of phylogenetic trees and population structures is developed.
Etymology
Like many geckos, this species can lose its tail when alarmed. Its call or chirp rather resembles the sound "gecko, gecko". However, this is an interpretation, and the sound may also be described as "tchak tchak tchak" (often sounded six to nine times in sequence). In Asia/Southeast Asia, notably Indonesia, Thailand, Singapore, and Malaysia, geckos have local names onomatopoetically derived from the sounds they make: Hemidactylus frenatus is called "chee chak" or "chi chak" (pr- chee chuck), said quickly. Also commonly spelled as "cicak" in Malay dictionaries. In the Philippines they are called "butiki" in Tagalog, "tiki" in Visayan, "alutiit" in Ilocano, and in Thailand "jing-jok" (Thai: จิ้งจก[29]). In Myanmar, they are called "အိမ်မြှောင် - ain-mjong" ( "အိမ် - ain" means "house" and "မြှောင် - mjong" means "stick to"). In some parts of India and in Pakistan they are called "chhipkali" (Urdu:چھپکلی, Hindi: छिपकली), from chhipkana, to stick. In Nepal they are called "vhitti" (Nepali: भित्ती) or "mausuli" (Nepali: माउसुली). In other parts of India they are called "kirli" (Punjabi: ਕਿੜਲੀ), "jhiti piti" (Oriya: ଝିଟିପିଟି), "zethi" (Assamese: জেঠী), "thikthikiaa" (Maithili: ठिकठिकिया), "paal" (Marathi: पाल), "gawli" or "palli" (Malayalam: ഗവ്ളി (gawli), പല്ലി (palli), Tamil: பல்லி (palli)), Telugu: బల్లి (balli), Kannada: ಹಲ್ಲಿ (halli), "ali" (Sylheti: ꠀꠟꠤ), "garoli" (Gujarati: ગરોળી). In West Bengal and Bangladesh they are called "tiktiki" (Bengali: টিকটিকি) as the sound is perceived as "tik tik tik". In Sri Lanka they are called "huna" in singular form (Sinhalese: හුනා). In Central America they are sometimes called "Limpia Casas" (Spanish: Housecleaners) because they reduce the amount of insects and other arthropods in their homes and also called 'qui-qui' because of the sound they make.
House geckos in captivity
House geckos can be kept as pets in a vivarium with a clean substrate, and typically require a heat source and a place to hide in order to regulate their body temperature, and a system of humidifiers and plants to provide them with moisture.
The species will cling to vertical or even inverted surfaces when at rest. In a terrarium they will mostly be at rest on the sides or on the top cover rather than placing themselves on plants, decorations or on the substrate, thus being rather conspicuous.
House geckos are also used as a food source for some snakes.
Cultural beliefs

Geckos are considered poisonous in many parts of the world. In Southeast Asia, geckos are believed to be carriers of good omen. In the Philippines, geckos making a ticking sound are believed to indicate an imminent arrival of a visitor or a letter.[30]
An elaborate system of predicting good and bad omens based on the sounds made by geckos, their movement and the rare instances when geckos fall from roofs has evolved over centuries in India.[31][32] In some parts of India, the sound made by geckos is considered a bad omen; while in parts of India, Assam, West Bengal, Bangladesh and Nepal, it is considered to be an endorsement of the truthfulness of a statement made just before, because the sound "tik tik tik" resembles the expression "thik thik thik", which in many Indian languages (e.g. Bengali) means "right right right", i.e., a three-fold confirmation. The cry of a gecko from an east wall as one is about to embark on a journey is considered auspicious, but a cry from any other wall is supposed to be inauspicious. A gecko falling on someone's right shoulder is considered good omen, but a bad omen if it drops on the left shoulder. In Punjab, it is believed that contact with the urine of a gecko will cause leprosy.[33] In some places in India, it is believed that watching a lizard on the eve of Dhanteras is a good omen or a sign of prosperity.
Notes
- Ota, H. & Whitaker, A.H. 2010. Hemidactylus frenatus. The IUCN Red List of Threatened Species 2010: e.T176130A7184890. https://dx.doi.org/10.2305/IUCN.UK.2010-4.RLTS.T176130A7184890.en. Downloaded on 08 June 2018.
- "ITIS Standard Report Page: Hemidactylus frenatus". ITIS Report. ITIS-North America. Retrieved 2009-06-29.
- Newbery, Brock; Jones, Darryl N. (2007). Lunney, Daniel (ed.). Presence of Asian House Gecko Hemidactylus frenatus across an urban gradient in Brisbane: influence of habitat and potential for impact on native gecko species. Pest or Guest: The Zoology of Overabundance. Royal Zoological Society of New South Wales. pp. 59–65. doi:10.7882/fs.2007.009. ISBN 9780980327212.
- Keim, Lauren (2002). The spatial distribution of the introduced Asian House Gecko (Hemidactylus frenatus) across suburban/forest edges (BSc Honors thesis). Unpublished Honours Thesis, Department of Zoology and Entomology, the University of Queensland.
- Marcellini, Dale (1971). "Activity Patterns of the Gecko Hemidactylus frenatus". Copeia. American Society of Ichthyologists and Herpetologists. 1971 (4): 631–635. doi:10.2307/1442631. ISSN 0045-8511. JSTOR 1442631.
- McKay, J. Lindley; Griffiths, Anthony D.; Crase, Beth (December 2009). "Distribution and habitat Use by Hemidactylus frenatus Duméril and Bibron (Gekkonidae) in the Northern Territory, Australia". The Beagle: Records of the Museums and Art Galleries of the Northern Territory. 25: 111–116.
- Gallina-Tessaro, Patricia; Ortega-Rubio, Alfredo; Alvarez-Cardenas, Sergio; Arnaud, Gustavo (1998). "Colonization of Socorro Island (Mexico), by the tropical house gecko Hemidactylus frenatus (Squamata:Gekkonidae)". Review of Tropical Biology. 47: 237–238.
- Rödder, Dennis; Solé, Micro; Böhme, Wolfgang (December 2008). "Predicting the potential distributions of two alien invasive Housegeckos (Gekkonidae: Hemidactylus frenatus, Hemidactylus mabouia)" (PDF). North-Western Journal of Zoology. 4 (2): 236–246.
- Farr, William L. 2011. Distribution of Hemidactylus frenatus in Mexico. Southwestern Naturalist 56 (2): 265-273
- Case, T; Bolger, D; Petren, K (March 1994). "Invasions and competitive displacement among house geckos in the tropical pacific". Ecology. 75 (2): 464–477. doi:10.2307/1939550. JSTOR 1939550. S2CID 43147742.
- Petren, K; Bolger, D; Case, T (January 15, 1993). "Mechanisms in the Competitive Success of an invading gecko over an asexual native". Science. 259 (5093): 354–358. doi:10.1126/science.259.5093.354. PMID 17832351.
- Petren, K; Case, T (1996). "An experimental demonstration of exploitation competition in an ongoing invasion". Ecology. 77 (1): 118–132. doi:10.2307/2265661. JSTOR 2265661.
- Dame, Elizabeth; Petren, Kenneth (2006). "Behavioural mechanisms of invasion and displacement in Pacific island geckos (Hemidactylus)". Animal Behaviour. 71 (5): 1165–1173. doi:10.1016/j.anbehav.2005.10.009.
- Cole, Nik; Jones, Carl; Harris, Stephen (2005). "The need for enemy-free space: The impact of an invasive gecko on island endemics". Biological Conservation. 125 (4): 467–474. doi:10.1016/j.biocon.2005.04.017.
- Hoskin, Conrad (2011). "The invasion and potential impact of the Asian House Gecko (Hemidactylus frenatus) in Australia". Austral Ecology. 36 (3): 240–251. doi:10.1111/j.1442-9993.2010.02143.x.
- Cogger, H. G. (1992). Reptiles and Amphibians of Australia. Chatswood, NSW: Reed Books.
- Tyler, M. J. (1961). "The diet and feeding habits of Hemidactylus frenatus (Dumeril and Bibron) (Reptilia: Gekkonidae) at Rangoon, Burma". Transactions of the Royal Society of South Australia. 84: 45–49.
- Cameron, S.F.; Wynn, M.L.; Wilson, R.S. (2013). "Sex-specific trade-offs and compensatory mechanisms: bite force and sprint speed pose conflicting demands on the design of geckos (Hemidactylus frenatus)" (PDF). The Journal of Experimental Biology. 216 (Pt 20): 3781–3789. doi:10.1242/jeb.083063. PMID 23821718.
- Snyder, G.K.; Weathers, W.W. (September 1976). "Physiological responses to temperature in the tropical lizard Hemidactylus frenatus (Sauria: Gekkonidae)". Herpetologica. 32 (3): 252–256. JSTOR 3891449.
- Licht, P; Dawson, W.R.; Shoemaker, V.H.; Main, A.R. (1966). "Observations on thermal relations of Western Australian Lizards". Copeia. 1966 (1): 97–110. doi:10.2307/1440766. JSTOR 1440766.
- Werner, Yehudah. "Do gravid females of oviparous gekkonid lizards maintain elevated body temperatures? Hemidactylus frenatus and Lepidodactylus lugubris on Oahu". Amphibia-Reptilia.
- Bustard, Robert (September 1970). "The population ecology of the Australian gekkonid lizard Heteronotia binoei in an exploited forest". Journal of Zoology. 162 (1): 31–42. doi:10.1111/j.1469-7998.1970.tb01255.x.
- Church, Gilbert. "The Reproductive Cycles of the Javanese House Geckos, Cosymbotus platyurus, Hemidactylus frenatus, and Peropus mutilatus". Copeia. 62: 262–269. doi:10.2307/1440888.
- Murphy-Walker, S.; Haley, S. R. (1996). "Functional Sperm Storage Duration in Female Hemidactylus Frenatus (Family Gekkonidae)". Herpetologica. 32: 365–373.
- Yamamoto, Yurie; Hidetoshi, OTA (2006). "Long-term functional sperm storage by a female common House Gecko, Hemidactylus Frenatus, from the Ryukyu Archipelago, Japan". Current Herpetology. 25: 39–40. doi:10.3105/1345-5834(2006)25[39:LFSSBA]2.0.CO;2.
- Darevsky, I.S.; Kupriyanova, L.A.; Roschchin, V.V. (1984). "A New All-Female Triploid Species of Gecko and Karyological Data on the Bisexual Hemidactylus frenatus from Vietnam". Journal of Herpetology. 18 (3): 277–284. doi:10.2307/1564081. JSTOR 1564081.
- King, Max (1978). "A New Chromosome Form of Hemidactylus Frenatus (Dumeril and Bibron)". Herpetologica. 34: 216–218.
- Storr, G.M. (1978). "Seven New Gekkonid Lizards from Western Australia". Records of the Western Australian Museum. 6.
- Tiyapan, Kittisak Nui (20 April 2018). Thai Grammar, Poetry and Dictionary, in a New Romanised System. Lulu.com. p. 102. ISBN 9789741718610 – via Google Books.
- "Mga Hayop (The Animals)". Northern Illinois University. Retrieved 2019-06-24.
- "ഗൗളിശാസ്ത്രം | Mashithantu | English Malayalam Dictionary മഷിത്തണ്ട് | മലയാളം < - > ഇംഗ്ലീഷ് നിഘണ്ടു". Dictionary.mashithantu.com. Retrieved 2013-12-14.
- "Hindu Omens". Oldandsold.com. Retrieved 2013-12-14.
- "The Folklore of Geckos: Ethnographic Date from South and West Asia". Nirc.nanzan-u.ac.jp. Retrieved 2014-02-02.
References
- Cook, Robert A. 1990 Range extension of the Darwin house gecko, Hemidactylus frenatus. Herpetofauna (Sydney) 20 (1): 23-27
- Darevsky, I S; Kupriyanova, L A; Roshchin, V V (1984). "A new all-female triploid species of gecko and karyological data on the bisexual Hemidactylus frenatus from Vietnam". Journal of Herpetology. 18 (3): 277–284. doi:10.2307/1564081.
- Edgren, Richard A (1950). "Notes on the Neotropical population of Hemidactylus frenatus". Schlegel Natural History Miscellanea. 55: 1–3.
- Jerdon, T.C. (1853). "Catalogue of the Reptiles inhabiting the Peninsula of India. Part 1". J. Asiat. Soc. Bengal. xxii: 462–479.
- McCoy, C. J.; Busack, Stephen D. (1970). "The lizards Hemidactylus frenatus and Leiolopisma metallica on the Island of Hawaii". Herpetologica. 26 (3): 303.
- Norman, Bradford R (2003). "A new geographical record for the introduced house gecko, Hemidactylus frenatus, at Cabo San Lucas, Baja California Sur, Mexico, with notes on other species observed". Bulletin of the Chicago Herpetological Society. 38 (5): 98–100. [erratum in 38(7):145]
- Ota, H. (1989). "Hemidactylus okinawensis Okada 1936, junior synonym of H. frenatus in Duméril & Bibron 1836". J. Herpetol. 23 (4): 444–445. doi:10.2307/1564064.
- Saenz, Daniel; Klawinski, Paul D (1996). "Geographic Distribution. Hemidactylus frenatus". Herpetological Review. 27 (1): 32.
External links
| The Wikibook Animal Care has a page on the topic of: House Gecko |


- Interaction of Asian House geckos and Wasps
- Gecko whoopee, introduced Asian House Geckos in Laguna de Apoyo Nature Reserve, Nicaragua