Chorismate synthase
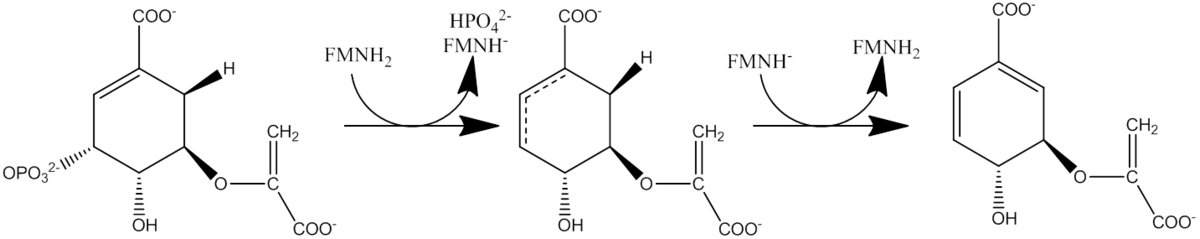
In enzymology, a chorismate synthase (EC 4.2.3.5) is an enzyme that catalyzes the chemical reaction
- 5-O-(1-carboxyvinyl)-3-phosphoshikimate chorismate + phosphate
| chorismate synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 4.2.3.5 | ||||||||
| CAS number | 9077-07-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Hence, this enzyme has one substrate, 5-O-(1-carboxyvinyl)-3-phosphoshikimate, and two products, chorismate and phosphate.
This enzyme belongs to the family of lyases, specifically those carbon-oxygen lyases acting on phosphates. The systematic name of this enzyme class is 5-O-(1-carboxyvinyl)-3-phosphoshikimate phosphate-lyase (chorismate-forming). This enzyme is also called 5-O-(1-carboxyvinyl)-3-phosphoshikimate phosphate-lyase. This enzyme participates in phenylalanine, tyrosine and tryptophan biosynthesis.
| Chorismate synthase | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||
| Symbol | Chorismate_synt | ||||||||||
| Pfam | PF01264 | ||||||||||
| InterPro | IPR000453 | ||||||||||
| PROSITE | PDOC00628 | ||||||||||
| SCOPe | 1q1l / SUPFAM | ||||||||||
| |||||||||||
Chorismate synthase catalyzes the last of the seven steps in the shikimate pathway which is used in prokaryotes, fungi and plants for the biosynthesis of aromatic amino acids. It catalyzes the 1,4-trans elimination of the phosphate group from 5-enolpyruvylshikimate-3-phosphate (EPSP) to form chorismate which can then be used in phenylalanine, tyrosine or tryptophan biosynthesis. Chorismate synthase requires the presence of a reduced flavin mononucleotide (FMNH2 or FADH2) for its activity. Chorismate synthase from various sources shows[2][3] a high degree of sequence conservation. It is a protein of about 360 to 400 amino-acid residues.
Biological and practical function
The shikimate pathway synthesises aromatic amino acids as well as other aromatic compounds that have various involvement with processes such as "UV protection, electron transport, signaling, communication, plant defense, and the wound response".[4] Moreover, the enzymes catalysing the Shikimate pathway may be potentially useful in the development of new herbicides and antibiotics. This is due to the fact that the shikimate pathway is not present in humans. It is, therefore, an ideal target for herbicides because chemicals used to inhibit the shikimate pathway should not have an effect on humans. Also, the products created by the shikimate pathway are essential to plant life, so, if the function of the pathway is inhibited, this has a fatal effect on the plant. The enzymes in the shikimate pathway, and chorismate synthase specifically,are also considered to be potential targets for new antimicrobial chemotherapy treatments for Mycobacterium tuberculosis. This is important since drugs resistant strains of M. tuberculosis continue to emerge. Studies have shown that the "shikimate pathway is essential for M. tuberculosis viability" making it an attractive target for new antimycobacterial agents.[5]
Structural studies
As of late 2007, 9 structures have been solved for this class of enzymes, with PDB accession codes 1Q1L, 1QXO, 1R52, 1R53, 1SQ1, 1UM0, 1UMF, 1ZTB, and 2G85.
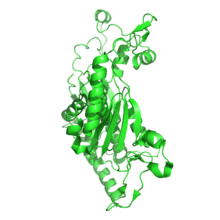
The crystal structure of chorismate synthase is a homotetramer with one FMN molecule non-covalently bound to each of the four monomers. Each monomer is made up of 9 alpha helices and 18 beta strands and the core is assembled in a unique beta-alpha-beta sandwich fold. The active sites for FMN-binding are made up of clusters of flexible loops and the area around these regions have highly positive electromagnetic potential. There are two histidine residues located at the active site which are thought to protonate the reduced flavin molecule and the leaving phosphate group of the substrate.[6]
 shows one of the four monomers that make up the active chorismate synthase molecule interacting with the FMN molecule in Mycobacterium tuberculosis |
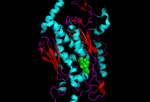 cartoon structure of one of the monomers present in the chorismate synthase moleucle with FMN molecule shown in green |
 this shows the interaction between the four monomers creating the chorismate synthase tetramer |
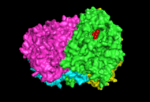 complete chorismate synthase tetramer shown interacting with one FMN molecule |
Mechanism
Reduced flavin (FMN) transfers an electron to the substrate resulting in cleavage of the C--O bond. How this flavin is obtained differs from one organism to another and the process is not completely understood.[4] Following the binding of EPSP, the flavin reaction intermediate is formed, but this process is completed prior to EPSP consumption. After EPSP has been converted to chorismate, and after the phosphate has been released from the enzyme,then the flavin intermediate will decay.[7] The chorismate synthase enzyme can be divided into two categories based on how reduced FMN is obtained. Bifunctional chorismate synthase is present in fungi and require a "second enzymatic activity, an NAD(P)H-dependent flavin reductase"."[5] Monofunctional chorismate synthase is found in plants and E.coli and is only active in an anaerobic environment with "chemically or enzymatically reduced flavin".[5] There is no net redox change in the rection. The flavin molecule is not consumed during the reaction and merely acts as a catalyst.
References
- 1ZTB Dias; et al. (2006). "Structure of chorismate synthase from Mycobacterium tuberculosis". Journal of Structural Biology. 154 (2): 130–143. doi:10.1016/j.jsb.2005.12.008. PMID 16459102.; rendered with PyMOL
- Schaller A, Schmid J, Leibinger U, Amrhein N (1991). "Molecular cloning and analysis of a cDNA coding for chorismate synthase from the higher plant Corydalis sempervirens Pers". J. Biol. Chem. 266 (32): 21434–21438. PMID 1718979.
- Braus GH, Reusser U, Jones DG (1991). "Molecular cloning, characterization and analysis of the regulation of the ARO2 gene, encoding chorismate synthase, of Saccharomyces cerevisiae". Mol. Microbiol. 5 (9): 2143–2152. doi:10.1111/j.1365-2958.1991.tb02144.x. PMID 1837329.
- Macheroux, P.; Schmid, J. R.; Amrhein, N.; Schaller, A. (1999). "A unique reaction in a common pathway: Mechanism and function of chorismate synthase in the shikimate pathway". Planta. 207 (3): 325–334. doi:10.1007/s004250050489. PMID 9951731.
- Ely, F.; Nunes, J. E.; Schroeder, E. K.; Frazzon, J.; Palma, M. S.; Santos, D. S.; Basso, L. A. (2008). "The Mycobacterium tuberculosis Rv2540c DNA sequence encodes a bifunctional chorismate synthase". BMC Biochemistry. 9: 13. doi:10.1186/1471-2091-9-13. PMC 2386126. PMID 18445278.
- Ahn, H. J.; Yoon, H. J.; Lee, B. I.; Suh, S. W. (2004). "Crystal Structure of Chorismate Synthase: A Novel FMN-binding Protein Fold and Functional Insights". Journal of Molecular Biology. 336 (4): 903–915. doi:10.1016/j.jmb.2003.12.072. PMID 15095868.
- Herrmann, K. M.; Weaver, L. M. (1999). "The Shikimate Pathway". Annual Review of Plant Physiology and Plant Molecular Biology. 50: 473–503. doi:10.1146/annurev.arplant.50.1.473. PMID 15012217.
- Gaertner FH, Cole KW (1973). "Properties of chorismate synthase in Neurospora crassa". J. Biol. Chem. 248 (13): 4602–9. PMID 4146266.
- Morell H, Clark MJ, Knowles PF, Sprinson DB (1967). "The enzymic synthesis of chorismic and prephenic acids from 3-enolpyruvylshikimic acid 5-phosphate". J. Biol. Chem. 242 (1): 82–90. PMID 4289188.
- Welch GR, Cole KW, Gaertner FH (1974). "Chorismate synthase of Neurospora crassa: a flavoprotein". Arch. Biochem. Biophys. 165 (2): 505–18. doi:10.1016/0003-9861(74)90276-8. PMID 4155270.
- Bornemann S, Lowe DJ, Thorneley RN (1996). "The transient kinetics of Escherichia coli chorismate synthase: substrate consumption, product formation, phosphate dissociation, and characterization of a flavin intermediate". Biochemistry. 35 (30): 9907–16. doi:10.1021/bi952958q. PMID 8703965.
- Bornemann S, Theoclitou ME, Brune M, Webb MR, Thorneley RN, Abell C (2000). "A Secondary beta Deuterium Kinetic Isotope Effect in the Chorismate Synthase Reaction". Bioorganic Chemistry. 28 (4): 191–204. doi:10.1006/bioo.2000.1174. PMID 11034781.
- Osborne A, Thorneley RN, Abell C, Bornemann S (2000). "Studies with substrate and cofactor analogues provide evidence for a radical mechanism in the chorismate synthase reaction". J. Biol. Chem. 275 (46): 35825–30. doi:10.1074/jbc.M005796200. PMID 10956653.
External links