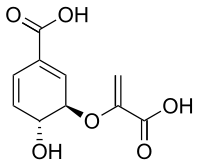
Chorismic acid
Chorismic acid, more commonly known as its anionic form chorismate, is an important biochemical intermediate in plants and microorganisms. It is a precursor for:
- The aromatic amino acids phenylalanine, tryptophan, and tyrosine
- Indole, indole derivatives and tryptophan
- 2,3-Dihydroxybenzoic acid (DHB) used for enterobactin biosynthesis
- The plant hormone salicylic acid[1]
- Many alkaloids and other aromatic metabolites.
- The folate precursor para-aminobenzoate (pABA)
- The biosynthesis of Vitamin K and folate in plants and microorganisms.
 | |
| Names | |
|---|---|
| IUPAC name
(3R,4R)-3-[(1-carboxyvinyl)oxy]-4-hydroxycyclohexa-1,5-diene-1-carboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.164.204 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H10O6 | |
| Molar mass | 226.184 g·mol−1 |
| Melting point | 140 °C (284 °F; 413 K) |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H302, H312, H315, H319, H332, H335, H350, H361 |
| P201, P202, P261, P264, P270, P271, P280, P281, P301+312, P302+352, P304+312, P304+340, P305+351+338, P308+313, P312, P321, P322, P330, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The name chorismic acid derives from a classical Greek word χωρίζω meaning "to separate",[2] because the compound plays a role as a branch-point in aromatic amino acid biosynthesis.[3]
Biosynthesis
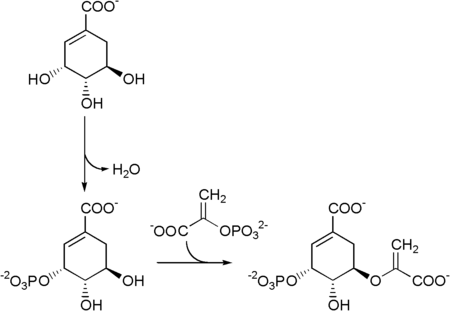
Shikimate → shikimate-3-phosphate → 5-enolpyruvylshikimate-3-phosphate (5-O-(1-carboxyvinyl)-3-phosphoshikimate)
Chorismate synthase is an enzyme that catalyzes the final chemical reaction:
- 5-O-(1-carboxyvinyl)-3-phosphoshikimate → chorismate + phosphate.
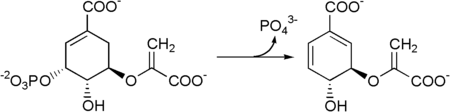
Metabolism
Chorismate is transformed into para-aminobenzoic acid by the enzymes 4-amino-4-deoxychorismate synthase and 4-amino-4-deoxychorismate lyase.
Chorismate lyase is an enzyme that transforms chorismate into 4-hydroxybenzoate and pyruvate. This enzyme catalyses the first step in ubiquinone biosynthesis in Escherichia coli and other Gram-negative bacteria.
See also
References
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001). "Isochorismate synthase is required to synthesize salicylic acid for plant defence". Nature. 414 (6863): 562–5. Bibcode:2001Natur.414..562W. doi:10.1038/35107108. PMID 11734859.
- Henry George Liddell; Robert Scott; Henry Stuart Jones & Roderick McKenzie. A Greek-English Lexicon. ISBN 0-19-864226-1.
- Gibson, F. (1999). "The elusive branch-point compound of aromatic amino acid biosynthesis". Trends in Biochemical Sciences. 24 (1): 36–38. doi:10.1016/S0968-0004(98)01330-9. PMID 10087921.