Chlorin
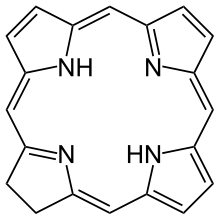
In organic chemistry, a chlorin is a tetrapyrrole. Chlorins are partially hydrogenated versions of porphyrins.[1] The parent chlorin is a rare compound, but substituted chlorins are common. Magnesium-containing chlorins are called chlorophylls. Chlorophylls are the photosensitive pigment in chloroplasts.
 | |
| Names | |
|---|---|
| Other names
2,3-Dihydroporphine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H16N4 | |
| Molar mass | 312.36784 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Related to chlorins are bacteriochlorins and isobacteriochlorins. They are found as the core of some bacteriochlorophylls. These tetrapyrroles are further reduced (hydrogenated) relative to chlorins.
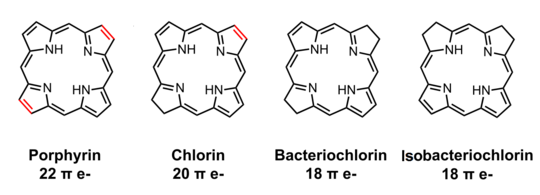
Structures comparing porphin, chlorin, bacteriochlorin, and isobacteriochlorin.
Because of their photosensitivity, chlorins are in active use as photosensitizing agents in experimental photodynamic therapy.[2]
See also
Further reading
- Juse´lius, Jonas; Sundholm, Dage (2000). "The aromatic pathways of porphins, chlorins and bacteriochlorins". Physical Chemistry Chemical Physics. 2 (10): 2145–2151. Bibcode:2000PCCP....2.2145J. doi:10.1039/b000260g.
References
- Gerard P. Moss (1988). "Nomenclature of Tetrapyrroles. Recommendations 1986". European Journal of Biochemistry. 178 (2): 277–328. doi:10.1111/j.1432-1033.1988.tb14453.x. PMID 3208761.
- Spikes, John D. (July 1990). "New trends in photobiology". Journal of Photochemistry and Photobiology B: Biology. 6 (3): 259–274. doi:10.1016/1011-1344(90)85096-F. PMID 2120404.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.