Chloroacetaldehyde
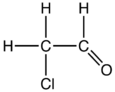
Chloroacetaldehyde is an organic compound with the formula ClCH2CHO. Like some related compounds, it is highly electrophilic reagent and a potentially dangerous alkylating agent. The compound is not normally encountered in the anhydrous form, but rather as the hemiacetal (ClCH2CH(OH))2O.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chloroacetaldehyde | |||
| Systematic IUPAC name
Chloroethanal | |||
| Other names
2-Chloroacetaldehyde 2-Chloroethanal | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.003.158 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H3ClO | |||
| Molar mass | 78.50 g mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | acrid, penetrating[1] | ||
| Density | 1.117 g/mL | ||
| Melting point | −16.3 °C (2.7 °F; 256.8 K) | ||
| Boiling point | 85 to 85.5 °C (185.0 to 185.9 °F; 358.1 to 358.6 K) | ||
| soluble[1] | |||
| Solubility | organic solvents | ||
| Hazards | |||
| Main hazards | alkylating agent | ||
| Flash point | 87.7 °C (189.9 °F) (closed cup) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
89 mg/kg (oral, rat) 82 mg/kg (oral, mouse)[2] | ||
LC50 (median concentration) |
200 ppm (rat, 1 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
C 1 ppm (3 mg/m3)[4] | ||
REL (Recommended) |
C 1 ppm (3 mg/m3)[4] | ||
IDLH (Immediate danger) |
45 ppm[4] | ||
| Related compounds | |||
Related compounds |
2-chloroethanol, Chloroacetic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chloroacetaldehyde is a useful intermediate in the synthesis of 2-aminothiazole[1] including the pharmaceuticals altizide, polythiazide, brotizolam and ciclotizolam.[5] Another use is to facilitate bark removal from tree trunks.[1]
Synthesis and reactions
Hydrated chloroacetaldehyde is produced by the chlorination of aqueous vinyl chloride:
- ClCH=CH2 + Cl2 + H2O → ClCH2CHO + 2 HCl
It can also be prepared from vinyl acetate[6] or by careful chlorination of acetaldehyde.[1] The related bromoacetaldehyde is prepared via bromination of vinyl acetate. It also rapidly forms an acetals in the presence of alcohols.[7]
Being bifunctional, chloroacetaldehyde is a versatile precursor to many heterocyclic compounds. It condenses with thiourea derivatives to give aminothiazoles. This reaction was important as a precursor to sulfathiazole, one of the first sulfa drugs.[6]
Anhydrous
Water free chloroacetaldehyde is prepared from the hydrate by azeotropic distillation with chloroform, toluene or carbon tetrachloride. Anhydrous chloroacetaldehyde reversibly converts to polyacetals.[5][1] Less reactive chloroacetaldehyde derivatives might be used instead to obtain chloroacetaldehyde or bypass its intermediate formation completely: e.g. chloroacetaldehyde dimethyl acetal (2-chloro-1,1-dimethoxyethane) hydrolyzes in acidic conditions to give chloroacetaldehyde, which may then quickly react with the other reagents[5] instead of polymerizing.
Hemihydrate
Hemihydrate is formed as below. It has a melting point of 43–50°C, boiling point of 85.5 °C.[1]

Environmental aspects
Chloroacetaldehyde is a metabolite in the degradation of 1,2-dichloroethane, which initially converts to chloroethanol. This metabolic pathway is topical because 1,2-dichloroethane is produced on a large as a precursor to vinyl chloride.[8]
Safety
Chloroacetaldehyde is corrosive to mucous membranes. It irritates eyes, skin and respiratory tract.[1]
Based on data collected from human studies in 1962, exposures to 45 ppm of chloroacetaldehyde were found to be disagreeable and caused conjunctival irritation to the subjects.[9] The Occupational Safety and Health Administration established a permissible exposure limit at a ceiling of 1 ppm (3 mg/m3) for exposures to chloroacetaldehyde.[10]
References
- The Merck index. S Budavari, M O'Neil, A Smith (12 ed.). Merck. 1996. p. 2108. ISBN 9780911910124.CS1 maint: others (link)
- "Chloroacetaldehyde". National Institute for Occupational Safety and Health. 4 December 2014. Retrieved 20 February 2015.
- "Chloroacetaldehyde". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- NIOSH Pocket Guide to Chemical Hazards. "#0118". National Institute for Occupational Safety and Health (NIOSH).
- Keiji, T (1992-10-30). "α-Chlorocarbonyl Compounds: Their Synthesis and Applications (Commemoration Issue Dedicated to Professor Shigeo Tanimoto On the Occation of His Retirement)". Bulletin of the Institute for Chemical Research, Kyoto University. 70 (3): 341. hdl:2433/77455. ISSN 0023-6071.
- Jira, Reinhard; Kopp, Erwin; McKusick, Blaine C.; Röderer, Gerhard; Bosch, Axel; Fleischmann, Gerald (2007). "Chloroacetaldehydes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_527.pub2.
- S. M. McElvain and D. Kundiger "Bromoacetal" Organic Syntheses 1943, volume 23, p. 8. doi:10.15227/orgsyn.023.0008.
- Janssen, D. B.; van der Ploeg, J. R. and Pries, F., "Genetics and Biochemistry of 1,2-Dichloroethane Degradation", Biodegradation, 1994, 5, 249-57.doi:10.1007/BF00696463
- Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs)
- CDC - NIOSH Pocket Guide to Chemical Hazards