Cerium(IV) oxide–cerium(III) oxide cycle
The cerium(IV) oxide–cerium(III) oxide cycle or CeO2/Ce2O3 cycle is a two-step thermochemical process that employs cerium(IV) oxide and cerium(III) oxide for hydrogen production.[1] The cerium-based cycle allows the separation of H2 and O2 in two steps, making high-temperature gas separation redundant.
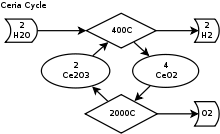
Simplified diagram of the cerium(IV) oxide–cerium(III) oxide cycle
Process description
The thermochemical two-step water splitting process (thermochemical cycle) uses redox systems:[2]
- Dissociation: 2CeO2 → Ce2O3 + 0.5 O2
- Hydrolysis: Ce2O3 + H2O → 2CeO2 + H2
For the first endothermic step, cerium(IV) oxide is thermally dissociated in an inert gas atmosphere at 2,000 °C (3,630 °F) and 100-200 mbar into cerium(III) oxide and oxygen. In the second exothermic step cerium(III) oxide reacts at 400 °C (752 °F)–600 °C (1,112 °F) in a fixed bed reactor with water and produces hydrogen and cerium(IV) oxide.
gollark: Have you tried direct intravenous caffeine?
gollark: What do you mean "hook" a panic?
gollark: You *can* always look at the 1894671829461824 lines of output LLVM or whatever to check!
gollark: https://www.reddit.com/r/rust/comments/4grfi3/argument_evaluation_order/
gollark: Hmm, apparently it's left to right.
See also
References
- "Hydrogen production from solar thermochemical water splitting cycles". Archived from the original on August 30, 2009.
- Steinfeld, Aldo; Haile, Sossina M.; Furler, Philipp; Scipio, Danien; Abbott, Mandy; Falter, Christoph; Chueh, William C. (December 24, 2010). "High-Flux Solar-Driven Thermochemical Dissociation of CO2 and H2O Using Nonstoichiometric Ceria". Science. 330 (6012): 1797–1801. doi:10.1126/science.1197834. PMID 21205663.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.