Hybrid sulfur cycle
The hybrid sulfur cycle (HyS) is a two-step water-splitting process intended to be used for hydrogen production. Based on sulfur oxidation and reduction, it is classified as a hybrid thermochemical cycle because it uses an electrochemical (instead of a thermochemical) reaction for one of the two steps. The remaining thermochemical step is shared with the sulfur-iodine cycle.
The HyS cycle was initially proposed and developed by Westinghouse Electric Corp. in the 1970s,[1] so it is also known as the "Westinghouse" cycle. Current development efforts in the United States are being led by the Savannah River National Laboratory.
Process Description
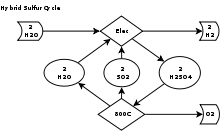
The two reactions in the HyS cycle are as follows:[2]
- H2SO4(aq) → H2O(g) + SO2(g) + ½ O2(g) (thermochemical, T > 800 °C)
- SO2(aq) + 2 H2O(l) → H2SO4(aq) + H2(g) (electrochemical, T = 80-120 °C)
- Net reaction: H2O(l) → H2(g) + ½ O2(g)
Sulfur dioxide acts to depolarize the anode of the electrolyzer. This results in a significant decrease in the reversible cell potential (and, therefore, the electric power requirement) for reaction (2). The standard cell potential for reaction (2) is -0.158 V at 298.15 K, compared to -1.229 V for the electrolysis of water (with oxygen evolution as the anodic reaction).[3]
See also
- Cerium(IV) oxide-cerium(III) oxide cycle
- Copper-chlorine cycle
- Iron oxide cycle
- Sulfur-iodine cycle
- Zinc zinc-oxide cycle
References
- Lee E. Brecher and Christopher K. Wu, “Electrolytic decomposition of water”, Westinghouse Electric Corp., Patent 3,888,750, June 10, 1975.
- Maximilian B. Gorensek & William A. Summers. Hybrid Sulfur flowsheets using PEM electrolysis and a bayonet decomposition reactor. International Journal of Hydrogen Energy. 2008;34(9):4097–4114. doi:10.1016/j.ijhydene.2008.06.049.
- Maximilian B. Gorensek, John A. Staser, Thomas G. Stanford & John W. Weidner. A thermodynamic analysis of the SO2/H2SO4 system in SO2-depolarized electrolysis. International Journal of Hydrogen Energy. 2009;34(15):6089–6095. doi:10.1016/j.ijhydene.2009.06.020.