Anostraca
Anostraca is one of the four orders of crustaceans in the class Branchiopoda; its members are also known as fairy shrimp. They are usually 6–25 mm (0.24–0.98 in) long (exceptionally up to 170 mm or 6.7 in). Most species have 20 body segments, bearing 11 pairs of leaf-like phyllopodia (swimming legs), and the body lacks a carapace. They live in vernal pools and hypersaline lakes across the world, including pools in deserts, in ice-covered mountain lakes and in Antarctica. They swim "upside-down" and feed by filtering organic particles from the water or by scraping algae from surfaces. They are an important food for many birds and fish, and some are cultured and harvested for use as fish food. There are 300 species spread across 8 families.
| Anostraca | |
|---|---|
 | |
| Artemia salina | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Crustacea |
| Class: | Branchiopoda |
| Subclass: | Sarsostraca Tasch, 1969 |
| Order: | Anostraca G. O. Sars, 1867 |
| Families [1][2] | |
| |
Description
The body of a fairy shrimp is elongated and divided into segments.[3] The whole animal is typically 6–25 millimetres (0.24–0.98 in) long, but one species, Branchinecta gigas does not reach sexual maturity until it reaches 50 mm (2.0 in) long, and can grow to 170 mm (6.7 in) long.[3] The exoskeleton is thin and flexible,[3] and lacks any sign of a carapace.[4] The body can be divided into three distinct parts (tagmata) – head, thorax and abdomen.[4]
Head
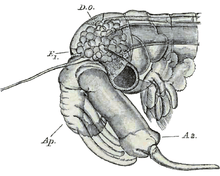
The head is morphologically distinct from the thorax. It bears two compound eyes on prominent stalks, and two pairs of antennae.[5] The first pair of antennae are small, usually unsegmented, and uniramous. The second pair are long and cylindrical in females, but in males they are enlarged and specialised for holding the female during mating.[5] In some groups, males have an additional frontal appendage.[5]
Thorax and abdomen

The thorax of most anostracans has 13 segments (19 in Polyartemiella and 21 in Polyartemia).[6] All but the last two are very similar, with a pair of biramous phyllopods (flattened, leaf-like appendages).[4] The last two segments are fused together,[3] and their appendages are specialised for reproduction.[6] Most anostracans have separate sexes (gonochorism), but a few reproduce by parthenogenesis.[7] The abdomen comprises 6 segments without appendages, and a telson,[6] which bears two flattened caudal rami or "cercopods".[3]
Internal anatomy
The head contains two digestive glands and the small lobate stomach that they empty into. This is connected to a long intestine, which terminates in a short rectum, with the anus located on the telson.[5] The haemocoel of anostracans is pumped by a long, tubular heart, which runs through most of the animal's length.[5] A series of slits allow haemocoel into the heart, which is then pumped out of the anterior opening by peristalsis.[5] The nervous system consists of two nerve cords which run the length of the body, with two ganglia and two transverse commissures in most of the body segments.[5]
Gas exchange is thought to take place through the entire body surface, but especially that of the phyllopodia and their associated gills, which may also be responsible for osmotic regulation.[5] Two coiled glands at the bases of the maxillae are used to excrete nitrogenous waste, typically in the form of urea.[5] Most of the animal's nitrogenous waste is, however, in the form of ammonia, which probably diffuses into the environment through the phyllopodia and gills.[5]
Ecology and behaviour
Anostracans inhabit inland waters ranging from hypersaline lakes to lakes that are almost devoid of dissolved substances;[3] they are "the most archetypal crustaceans" in ephemeral waters.[8] The relatively large size of fairy shrimp, together with their slow means of locomotion, makes them an easy target for predatory fish and waterfowl.[8] This has led to their distribution being restricted to environments with fewer predators, such as vernal pools, salt lakes and lakes at high altitudes or latitudes.[8] The southernmost recorded fairy shrimp is Branchinecta gaini from the Antarctic Peninsula,[9] while the altitude record is held by B. brushi, which lives at 5,930 metres (19,460 ft) in the Chilean Andes.[10] Other genera, such as Streptocephalus, occur in deserts throughout the world.[11]
Anostracans swim gracefully by movements of their phyllopodia (thoracic appendages) in a metachronal rhythm.[5] When swimming, the animal's ventral side is normally uppermost (often described as swimming "upside-down").[3] They filter food indiscriminately from the water as they swim, but also scrape algae and other organic materials from solid surfaces, for which they turn to have their ventral side against the food surface.[3]
Another important aspect of the fairy shrimp’s life cycle is their universal ability to enter diapause,[12][13] a state of biological dormancy where growth and metabolism are arrested,[14] as an egg (or cyst). This trait assists in both species' dispersal and in overcoming adverse environmental conditions.[13][14] Once dormant, these cysts can withstand conditions as harsh and diverse as droughts, frosts, hypersalinity, complete desiccation, exposure to UV radiation and the vacuum of space.[15][3][14] It is also the only way for the fairy shrimps to colonize new habitats—facilitated by a variety of conditions including wind, predators, currents[16][17][18]—as the soft-bodied adults are unable to leave the freshwater system.[17] Once in diapause, these cysts can remain viable for centuries,[16] and the mixing of system sediment results in the hatching of different aged cysts in each generation.[19][17][20] This inbreeding slows the rate of selection by resisting gene flow and minimizing phenotypic variation, in turn promoting the stability of the existing, successful phenotype.[17]
Anostracans are an important food source for many birds and fish. For example, they provide much of the food for female pintails and mallards in the Prairie Pothole Region of the Great Plains in North America, especially in years when temporary wetlands are abundant.[21] Similarly, Artemia forms an important part of the diet of flamingos wherever it can be found.[22]
Uses

Brine shrimp are used as food for fish and other organisms in aquaria and aquaculture.[23] Their drought-resistant eggs are collected from lakeshores and are stored and transported dry. They hatch readily when submerged in salt water. This is a multimillion-dollar industry, centred on the Great Salt Lake in Utah and San Francisco Bay in California;[24] adults are collected from Mono Lake and transported frozen.[23]
Fossil record and evolution
Fairy shrimp are believed to have diverged from the main line of Branchiopoda during the Ordovician period,[25][26] around the same time it is thought they colonized freshwater and estuarine ecosystems.[14] This transition is believed to have been the result of selection pressure to escape predation in the Early Paleozoic seas.[27][3][13]
Some studies point to fossils resembling fairy shrimp in the Upper Cambrian,[28][29] specifically the oldest known branchiopod fossil, Rebachiella kinnekullensis, from Orsten marine deposits.[30] Despite its seeming resemblance to modern fairy shrimp, this fossil is still considered by most to be an outlying member of the ancestral marine Branchiopoda rather than a true fairy shrimp.[25]
The monophyly of this order is well supported,[27][31][32][25][28][33][34] and the scientific community has reached consensus that Anostraca was the first group to branch off from the Branchiopoda.[12][28][13][34][26]
The radiation hypothesis championing rapid spread and colonization during the Gondwana fragmentation closely echoes the current distribution of the order.[26][17] Presently, Anostraca are found on all seven continents.[27] Most of the extant genera have restricted geographical distributions. Only three genera are widespread across the remnants of the former supercontinent Pangaea: Artemia, Branchinella and Branchinecta, while the remaining genera are found only throughout former Laurasia.[35] This suggests that much of the potential habitat in this supercontinent, now occupied by Anostraca, to have been unoccupied by ecologically similar species, or to have been inhabited by species with less adaptive ability.[17] Studies have found Anostraca capable of rapid colonization[36] and speciation.[19]
Diversity
.jpg)
Anostraca is the most diverse of the four orders of Branchiopoda. It comprises around 300 species, grouped into 26 genera in eight families:[27]
- Artemiidae – 1 genus, 9 species
- Branchinectidae – 1 genus, 45 species
- Branchipodidae – 5 genera, 35 species
- Chirocephalidae – 9 genera, 81 species
- Parartemiidae – 1 genus, 13 species
- Streptocephalidae – 1 genus, 56 species
- Tanymastigidae – 2 genera, 8 species
- Thamnocephalidae – 6 genera, 62 species
References
- Joel W. Martin; George E. Davis (2001). An Updated Classification of the Recent Crustacea (PDF). Natural History Museum of Los Angeles County. pp. 1–132.
- Peter H. H. Weekers; Gopal Murugan; Jacques R. Vanfleteren; Denton Belk; Henri J. Dumont (2002). "Phylogenetic analysis of anostracans (Branchiopoda: Anostraca) inferred from nuclear 18S ribosomal DNA (18S rDNA) sequences" (PDF). Molecular Phylogenetics and Evolution. 25 (3): 535–544. doi:10.1016/S1055-7903(02)00289-0. PMID 12450757.
- Denton Belk (2007). "Branchiopoda". In Sol Felty Light; James T. Carlton (eds.). The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon (4th ed.). University of California Press. pp. 414–417. ISBN 978-0-520-23939-5.
- William David Williams (1980). "Arachnids and Crustaceans". Australian Freshwater Life: the Invertebrates of Australian Inland Waters (2nd ed.). Palgrave Macmillan Australia. pp. 118–184. ISBN 978-0-333-29894-7.
- Douglas Grant Smith (2001). "Phyllopodous Branchiopoda (fairy, tadpole, and clam shrimps)". In Douglas Grant Smith (ed.). Pennak's Freshwater Invertebrates of the United States: Porifera to Crustacea (4th ed.). John Wiley and Sons. pp. 427–452. ISBN 978-0-471-35837-4.
- D. R. Khanna (2004). "Segmentation in arthropods". Biology of Arthropoda. Discovery Publishing House. pp. 316–394. ISBN 978-81-7141-897-8.
- Graham Bell (1982). "Arthropoda: Crustacea Branchiopoda". The Masterpiece of Nature: the Evolution and Genetics of Sexuality. Cambridge University Press. pp. 239–248. ISBN 978-0-85664-753-6.
- Henri J. Dumont (2009). "The crustacean zooplankton (Copepoda, Branchiopoda), atyid Decapoda, and Syncarida of the Nile Basin". In Henri J. Dumont (ed.). The Nile: Origins, Environments, Limnology and Human Use. Volume 89 of Monographiae Biologicae. Springer. pp. 521–546. ISBN 978-1-4020-9725-6.
- T.C. Hawes (October 2009). "Origins and dispersal of the Antarctic fairy shrimp". Antarctic Science. 21 (5): 477–482. doi:10.1017/S095410200900203X.
- Thomas A. Hegna; Eric A. Lazo-Wasem (1 July 2010). "Branchinecta brushi n. sp. (Branchiopoda: Anostraca: Branchinectidae) from a volcanic crater in northern Chile (Antofagasta Province): a new altitude record for crustaceans" (PDF). Journal of Crustacean Biology. 30 (3): 445–464. doi:10.1651/09-3236.1.
- David Ward (2009). "Biodiversity and biogeography of deserts". The Biology of Deserts. Oxford University Press. pp. 192–216. ISBN 978-0-19-921147-0.
- Hairston, Nelson G.; Cáceres, Carla E. (1996-03-01). "Distribution of crustacean diapause: micro- and macroevolutionary pattern and process". Hydrobiologia. 320 (1–3): 27–44. doi:10.1007/bf00016802. ISSN 0018-8158.
- Fryer, Geoffrey (1996-03-01). "Diapause, a potent force in the evolution of freshwater crustaceans". Hydrobiologia. 320 (1–3): 1–14. doi:10.1007/bf00016800. ISSN 0018-8158.
- Alekseev, Victor R.; Starobogatov, Yaroslav I. (1996-03-01). "Types of diapause in Crustacea: definitions, distribution, evolution". Hydrobiologia. 320 (1–3): 15–26. doi:10.1007/bf00016801. ISSN 0018-8158.
- Czyż, M.; Woliński, P.; Gołdyn, B. (2016). "Cyst morphology of large branchiopod crustaceans (Anostraca, Notostraca, Laevicaudata, Spinicaudata) in western Poland". Biological Letters. 53 (2): 79–88. doi:10.1515/biolet-2017-0006.
- Parnov, V.; Krylov, P.; Riccardi, N. (2004). "Role of diapause in dispersal and invasion success by aquatic invertebrates". Journal of Limnology. 63: 59–69.
- Rogers, C. (2015). "A conceptual model for Anostracan biogeography". Journal of Crustacean Biology. 35 (5): 686–699. doi:10.1163/1937240x-00002369.
- Brendonck, Luc; Riddoch, Bruce J. (1999-05-01). "Wind-borne short-range egg dispersal in anostracans (Crustacea: Branchiopoda)". Biological Journal of the Linnean Society. 67 (1): 87–95. doi:10.1111/j.1095-8312.1999.tb01931.x. ISSN 0024-4066.
- Remigio, E. A.; Hebert, P. D. N.; Savage, A. (2001-09-01). "Phylogenetic relationships and remarkable radiation in Parartemia (Crustacea: Anostraca), the endemic brine shrimp of Australia: evidence from mitochondrial DNA sequences". Biological Journal of the Linnean Society. 74 (1): 59–71. doi:10.1006/bijl.2001.0567. ISSN 0024-4066.
- Kraus, Holger; Eder, Erich; Sten Møller, Ole; Werding, Bernd (2004-07-01). "Cyst Deposition Behaviour and the Functional Morphology of the Brood Pouch in Streptocephalus Torvicornis (Branchiopoda: Anostraca)". Journal of Crustacean Biology. 24 (3): 393–397. doi:10.1651/c-2470. ISSN 0278-0372.
- Gary L. Krapu; Kenneth J. Reinecke (1992). "Foraging ecology and nutrition". In Bruce D. J. Batt (ed.). Ecology and Management of Breeding Waterfowl. University of Minnesota Press. pp. 1–29. ISBN 978-0-8166-2001-2.
- Simon Aspinall; Peter Hellyer (2002). "Saline wetland reserve management: a case study from the United Arab Emirates". In Hans-Jörg Barth; Benno Böer (eds.). Sabkha Ecosystems, Volume 2. Tasks for Vegetation science. Springer. pp. 335–340. ISBN 978-1-4020-0504-6.
- J. M. Melack (2009). "Saline and soda lakes". In Sven Erik Jørgensen (ed.). Ecosystem Ecology. Academic Press. pp. 380–384. ISBN 978-0-444-53466-8.
- Hugh F. Clifford (1991). "Anostraca". Aquatic Invertebrates of Alberta: an Illustrated Guide. University of Alberta. pp. 140–143. ISBN 978-0-88864-234-9.
- Olesen, Jørgen (2007-04-01). "Monophyly and Phylogeny of Branchiopoda, with Focus on Morphology and Homologies of Branchiopod Phyllopodous Limbs". Journal of Crustacean Biology. 27 (2): 165–183. doi:10.1651/s-2727.1. ISSN 0278-0372.
- Daniels, Savel R.; Hamer, Michelle; Rogers, Christopher (2004-07-01). "Molecular evidence suggests an ancient radiation for the fairy shrimp genus Streptocephalus (Branchiopoda: Anostraca)". Biological Journal of the Linnean Society. 82 (3): 313–327. doi:10.1111/j.1095-8312.2004.00359.x. ISSN 0024-4066.
- Luc Brendonck; D. Christopher Rogers; Jorgen Olesen; Stephen Weeks; Walter R. Hoch (2008). "Global diversity of large branchiopods (Crustacea: Branchiopoda) in freshwater". In Estelle V. Balian; Christian Lévêque; Hendrik Segers; Koen Martens (eds.). Freshwater Animal Diversity Assessment. Developments in Hydrobiology 198. pp. 167–176. doi:10.1007/s10750-007-9119-9. ISBN 978-1-4020-8258-0. Reprinted from Hydrobiologia, Volume 595.
- Richter, Stefan; Olesen, Jørgen; Wheeler, Ward C. (2007-08-01). "Phylogeny of Branchiopoda (Crustacea) based on a combined analysis of morphological data and six molecular loci". Cladistics. 23 (4): 301–336. doi:10.1111/j.1096-0031.2007.00148.x. ISSN 1096-0031.
- Brendonck, L. (1996). "Diapause, quiescence, hatching requirements: what we can learn from large freshwater branchiopods (Crustacea Branciopoda: Anostraca, Notostraca, Conchostraca)". Hydrobiologia. 320 (1–3): 85–97. doi:10.1007/bf00016809.
- Joel W. Martin; Michael S. Laverack (December 1992). "On the distribution of the crustacean dorsal organ" (PDF). Acta Zoologica. 73 (5): 357–368. doi:10.1111/j.1463-6395.1992.tb01108.x. Archived from the original (PDF) on 2011-07-19.
- Fortey, R.; Thomas, H. (2012). Arthropod Relationships. Berlin: Springer Science & Business Media. pp. 104–105.
- Minelli, A. (2009). Perspectives in Animal Phylogeny and Evolution. Oxford: Oxford University Press. pp. 98–100.
- Weekers, P (2002). "Phylogenetic analysis of anostracans (Branchiopoda: Anostraca) inferred from nuclear 18S ribosomal DNA (18S rDNA) sequences". Molecular Phylogenetics and Evolution. 25 (3): 535–544. doi:10.1016/s1055-7903(02)00289-0. PMID 12450757.
- Regier, Jerome C.; Shultz, Jeffrey W.; Zwick, Andreas; Hussey, April; Ball, Bernard; Wetzer, Regina; Martin, Joel W.; Cunningham, Clifford W. (2010-02-10). "Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences". Nature. 463 (7284): 1079–1083. doi:10.1038/nature08742. ISSN 1476-4687. PMID 20147900.
- D. Dudley Williams (1987). "The Biota". The Ecology of Temporary Waters. Taylor & Francis. pp. 21–67. ISBN 978-0-7099-5211-4.
- Kappas, Ilias; Mura, Graziella; Synefiaridou, Dimitra; Marrone, Federico; Alfonso, Giuseppe; Alonso, Miguel; Abatzopoulos, Theodore J. (2017-10-01). "Molecular and morphological data suggest weak phylogeographic structure in the fairy shrimp Streptocephalus torvicornis (Branchiopoda, Anostraca)". Hydrobiologia. 801 (1): 21–32. doi:10.1007/s10750-017-3203-6. ISSN 0018-8158.
