Alpha-particle spectroscopy
One method for testing of (and measuring) many alpha emitters is to use alpha-particle spectroscopy. For methods of gamma rays and beta particles, please see gamma spectroscopy and liquid scintillation counting respectively.
Experimental methods
Counting using a source in a metal disk
It is common to place a drop of the test solution on a metal disk which is then dried out to give a uniform coating on the disk. This is then used as the test sample. If the thickness of the layer formed on the disk is too thick then the lines of the spectrum are broadened to lower energies. This is because some of the energy of the alpha particles is lost during their movement through the layer of active material.
Liquid scintillation
An alternative method is to use internal liquid scintillation counting, where the sample is mixed with a scintillation cocktail. When the light emissions are then counted, some machines will record the amount of light energy per radioactive decay event. Due to the imperfections of the liquid scintillation method (such as a failure for all the photons to be detected, cloudy or coloured samples can be difficult to count) and the fact that random quenching can reduce the number of photons generated per radioactive decay, it is possible to get a broadening of the alpha spectra obtained through liquid scintillation. It is likely that these liquid scintillation spectra will be subject to a Gaussian broadening, rather than the distortion exhibited when the layer of an active material on a disk is too thick.
Alpha spectra
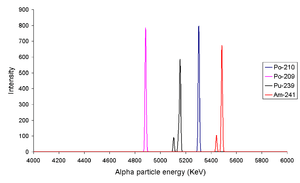
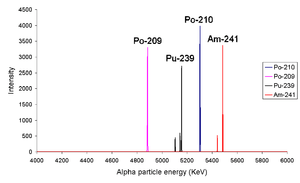
From left to right the peaks are due to 209Po, 210Po, 239Pu and 241Am. The fact that isotopes such as 239Pu and 241Am have more than one alpha line indicates that the nucleus has the ability to be in different discrete energy levels.
Calibration: MCA does not work on energy, it works on voltage. To relate the energy to voltage one must calibrate the detection system. Here different alpha emitting sources of known energy were placed under the detector and the full energy peak is recorded.
Measurement of thickness of thin foils: Energies of alpha particles from radioactive sources are measured before and after passing through the thin films. By measuring difference and using SRIM we can measure the thickness of thin foils.
Energetics of alpha decay: The alpha particle, or 4He nucleus, is an especially strongly bound particle. This combined with the fact that the binding energy per nucleon has a maximum value near A»56 and systematically decreases for heavier nuclei, creates the situation that nuclei with A>150 have positive Qα-values for the emission of alpha particles.
For example, one of the heaviest naturally occurring isotopes, 238U (with a mass excess, Δ, of +47.3070 MeV) decays by alpha emission to 234Th (Δ = +40.612 MeV) giving a Q-value:
- Qα = 47.3070 - (40.612 + 2.4249) = 4.270 MeV
Note that the decay energy will be divided between the alpha-particle and the heavy recoiling daughter so that the kinetic energy of the alpha particle will be slightly less. The kinetic energy of the recoiling 234Th nucleus produced in the decay of 238U is ~0.070 MeV. Conservation of momentum and energy in this reaction requires that the kinetic energy of the alpha-particle, Tα, is equal in magnitude.
The kinetic energies of the emitted alpha particles can be measured very precisely so we should be careful to distinguish between the Qα‐value and the kinetic energy, Tα. The very small recoil energy of the heavy daughter is very difficult to measure but it is still large compared to chemical bond energies and can lead to interesting chemistry. For example, the daughter nuclei may recoil out of the original alpha-source. This can cause serious contamination problems if the daughters are themselves radioactive. The Qα‐values generally increase with increasing atomic number but the variation in the mass surface due to shell effects can overwhelm the systematic increase. The sharp peaks near A=214 are due to the effects of the N=126 shell.