Acyl azide
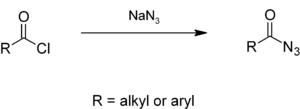
Acyl azides are carboxylic acid derivatives with the general formula RCON3.
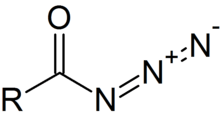
A general acyl azide
Preparation
Alkyl or aryl acyl chlorides react with sodium azide to give acyl azides.[1][2]
They can also be synthesized from various carboxylic acids and sodium azide in presence of triphenylphosphine and trichloroacetonitrile catalysts in excellent yields at mild conditions.[3] Another route starts with aliphatic and aromatic aldehydes reacting with iodine azide which is formed from sodium azide and iodine monochloride in acetonitrile.[4]
Uses
Acyl azides are used as chemical reagents. On Curtius rearrangement acyl azides yield isocyanates.[5][6][7][8]
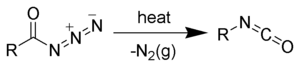
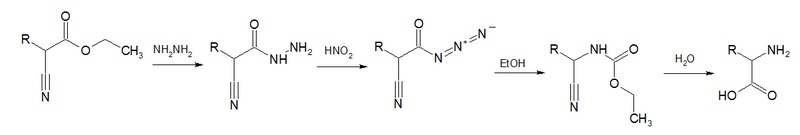
Acyl azides are also formed in Darapsky degradation,[9][10][11][12][13]
gollark: .·
gollark: ++tel init_webhook
gollark: !np
gollark: MUTE THE CHANNEL, BEE
gollark: * suppose
References
- Allen, C. F. H.; Bell, Alan (1944). "Undecyl isocyanate". Organic Syntheses. 24: 94. doi:10.15227/orgsyn.024.0094.; Collective Volume, 3, p. 846
- Munch-Petersen, Jon (1953). "m-Nitrobenzazide (Benzoyl azide, m-nitro-)". Organic Syntheses. 33: 53. doi:10.15227/orgsyn.033.0053.; Collective Volume, 4, p. 715
- Jang, Doo; Kim, Joong-Gon (2008). "Direct Synthesis of Acyl Azides from Carboxylic Acids by the Combination of Trichloroacetonitrile, Triphenylphosphine and Sodium Azide". Synlett. 2008 (13): 2072–2074. doi:10.1055/s-2008-1077979.
- Marinescu, Lavinia; Thinggaard, Jacob; Thomsen, Ib B.; Bols, Mikael (2003). "Radical Azidonation of Aldehydes". J. Org. Chem. 68 (24): 9453–9455. doi:10.1021/jo035163v. PMID 14629171.
- Curtius, Th. (1890). "Ueber Stickstoffwasserstoffsäure (Azoimid) N3H". Ber. (in German). 23 (2): 3023–3033. doi:10.1002/cber.189002302232.
- Curtius, Th. (1894). "20. Hydrazide und Azide organischer Säuren I. Abhandlung". J. Prakt. Chem. (in German). 50 (1): 275–294. doi:10.1002/prac.18940500125.
- Smith, Peter A. S. (1946). "The Curtius reaction". Org. React. 3: 337–449. doi:10.1002/0471264180.or003.09. ISBN 0471264180.
- Scriven, Eric F. V.; Turnbull, Kenneth (1988). "Azides: Their preparation and synthetic uses". Chem. Rev. 88 (2): 297–368. doi:10.1021/cr00084a001.
- Darapsky, August (1936). "Darstellung von α-Aminosäuren aus Alkyl-cyanessigsäuren". J. Prakt. Chem. (in German). 146 (8–12): 250–267. doi:10.1002/prac.19361460806.
- Darapsky, August; Hillers, Dietrich (1915). "Über das Hydrazid der Cyanessigsäure, Isonitrosocyanessigsäure und Nitrocyanessigsäure". J. Prakt. Chem. (in German). 92 (1): 297–341. doi:10.1002/prac.19150920117.
- Gagnon, Paul E.; Boivin, Paul A.; Craig, Hugh M. (1951). "Synthesis of Amino Acids from Substituted Cyanoacetic Esters". Can. J. Chem. 29 (1): 70–75. doi:10.1139/v51-009.
- E. H. Rodd (1965). "Chemistry of Carbon Compounds" (2nd ed.). New York: 1157. Cite journal requires
|journal=(help) - Gagnon, Paul E.; Nadeau, Guy; Côté, Raymond (1952). "Synthesis of α-Amino Acids from Ethyl Cyanoacetate". Can. J. Chem. 30 (8): 592–597. doi:10.1139/v52-071.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.