Acryloyl group
In organic chemistry, the acryloyl group is a form of enone with structure H2C=CH–C(=O)–; it is the acyl group derived from acrylic acid. The preferred IUPAC name for the group is prop-2-enoyl, and it is also known as acrylyl or simply (and incorrectly) as acryl. Compounds containing an acryloyl group can be referred to as "acrylic compounds".
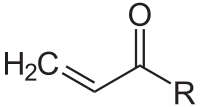
Structure of the acryloyl group
An acrylic compound is typically an α,β-unsaturated carbonyl compound: it contains a carbon–carbon double bond and a carbon–oxygen double bond (carbonyl) separated by a carbon–carbon single bond, thus possessing properties characteristic for both functional groups :
- at the C=C bond: electrophilic addition of acids and halogens, hydrogenation, hydroxylation and cleavage of the bond
- at the C=O bond: nucleophilic substitution (such as in esters) or nucleophilic addition (such as in ketones). The carboxyl group of acrylic acid can react with ammonia to form acrylamide, or with an alcohol to form an acrylate ester.
In addition, since both double bonds are separated by a single C–C bond, the double bonds are conjugated.
See also
- Acrylic polymer
Literature
- Klein, David R. (2012). Organic Chemistry. Hoboken, N.J: John Wiley. ISBN 9780471756149.
- Bruice, Paula Yurkanis (2010). Essential Organic Chemistry (2 ed.). Boston: Prentice Hall. ISBN 9780321644169.
- McMurry, John; McMurry, Susan (2012). Organic Chemistry (8 ed.). Belmont, CA: Brooks/Cole. ISBN 0840054556.
- Chang, Raymond (2008). General chemistry : the essential concepts (5 ed.). Boston: McGraw-Hill. ISBN 9780071102261.
- Chang, Raymond; Goldsby, Kenneth A. (2014). General chemistry : the essential concepts (7 ed.). New York: McGraw-Hill. ISBN 9781259060427.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.