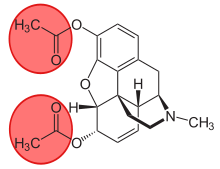
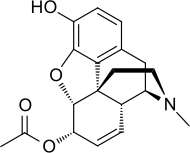
6-Monoacetylmorphine
6-Monoacetylmorphine (6-MAM, 6-acetylmorphine, or 6-AM) is one of three active metabolites of heroin (diacetylmorphine), the others being morphine and the much less active 3-monoacetylmorphine (3-MAM).
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 6-acetylmorphine |
| Routes of administration | Intravenous |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | < 5 mins |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.150.555 |
| Chemical and physical data | |
| Formula | C19H21NO4 |
| Molar mass | 327.380 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pharmacology
6-MAM occurs as a metabolite of heroin. Once it has passed first-pass metabolism, 6-MAM is then metabolized into morphine or excreted in urine.
Heroin is rapidly metabolized by esterase enzymes in the brain and has an extremely short half-life. It has also relatively weak affinity to μ-opioid receptors because the 3-hydroxy group, essential for effective binding to the receptor, is masked by the acetyl group. Therefore, heroin acts as a pro-drug, serving as a lipophilic transporter for the systemic delivery of morphine, which actively binds with μ-opioid receptors.[1][2]
6-MAM already has a free 3-hydroxy group and shares the high lipophilicity of heroin, so it penetrates the brain just as quickly and does not need to be deacetylated at the 6-position in order to be bioactivated; this makes 6-MAM somewhat more potent than heroin.[3]
Availability
6-MAM is rarely encountered in an isolated form due to the difficulty in selectively acetylating morphine at the 6-position without also acetylating the 3-position. However, it is found in significant amounts in black tar heroin along with heroin itself.
Synthesis
The production of black tar heroin results in significant amounts of 6-MAM in the final product. 6-MAM is approximately 30 percent more active than diacetylmorphine itself, This is why despite lower heroin content, black tar heroin may be more potent than some other forms of heroin. 6-MAM can be synthesized from morphine using glacial acetic acid with an organic base as a catalyst. The acetic acid must be of a high purity (97–99 per cent) for the acid to properly acetylate the morphine at the 6th position effectively creating 6-MAM. Acetic acid is used rather than acetic anhydride, as acetic acid is not strong enough to acetylate the phenolic 3-hydroxy group but is able to acetylate the 6-hydroxy group, thus selectively producing 6-MAM rather than heroin. Acetic acid is a convenient way to produce 6-MAM, as acetic acid also is not a watched chemical as it is the main component of vinegar.
Chemistry
Detection in bodily fluids
Since 6-MAM is a metabolite unique to heroin, its presence in the urine confirms heroin use. This is significant because a urine immunoassay drug screen typically tests for morphine, which is a metabolite of a number of legal and illegal opiates/opioids such as codeine, morphine sulfate, and heroin. Trace amounts of 6-MAM, a specific metabolite of heroin, are also excreted for approximately 6–8 hours following heroin use.[4] so a urine specimen must be collected soon after the last heroin use; however, the presence of 6-MAM suggests that heroin was used as recently as within the last day.
6-MAM is naturally found in trace amounts in the brain of certain mammals.[5]
See also
- M3G, morphine-3-glucuronide an inactive metabolite of morphine much as 3-MAM is the less active metabolite of heroin (notably here as morphine is an active secondary metabolite of heroin itself with 6-Monoacetylmorphine being the intermediate stage)
- M6G, morphine-6-glucuronide the active variant in close relation to 6-MAM, being relative as twin metabolites of this articles very metabolite itself, morphine, twinned to a metabolite (3-MAM) of a parent compound (heroin) of this article's chemical
References
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ (1983). "Evidence from opiate binding studies that heroin acts through its metabolites". Life Sciences. 33 Suppl 1: 773–6. doi:10.1016/0024-3205(83)90616-1. PMID 6319928.
- "Pagina di transizione". www.researchitaly.it.
- Tasker RA, Vander Velden PL, Nakatsu K (1984). "Relative cataleptic potency of narcotic analgesics, including 3,6-dibutanoylmorphine and 6-monoacetylmorphine". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 8 (4–6): 747–50. doi:10.1016/0278-5846(84)90051-4. PMID 6543399. S2CID 23566872.
- "Opiates | Drug Info | Resources | Redwood Toxicology Laboratory". www.redwoodtoxicology.com.
- Weitz CJ, Lowney LI, Faull KF, Feistner G, Goldstein A (July 1988). "6-Acetylmorphine: a natural product present in mammalian brain". Proceedings of the National Academy of Sciences of the United States of America. 85 (14): 5335–8. Bibcode:1988PNAS...85.5335W. doi:10.1073/pnas.85.14.5335. PMC 281745. PMID 3393541.