Prodine
Prodine (trade names Prisilidine and Nisentil) is an opioid analgesic that is an analog of pethidine (meperidine). It was developed in Germany in the late 1940s.
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
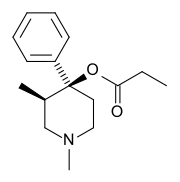
| Formula | C16H23NO2 |
| Molar mass | 261.365 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
There are two isomers of the trans form of prodine, alphaprodine and betaprodine. Both exhibit optical isomerism and alphaprodine and betaprodine are racemates.[1] Alphaprodine is closely related to desomorphine in steric configuration.[1] The cis form also has active isomers but none are used in medicine.[1][2] Betaprodine is some five times more potent than alphaprodine,[3] but is metabolized more rapidly, and only alphaprodine was developed for medicinal use. It has similar activity to pethidine, but with a faster onset of action and shorter duration.[4] Betaprodine produces more euphoria and side effects than alphaprodine at all dose levels, and it was found that 5 to 10 mg of betaprodine is equivalent to 25 to 40 mg of alphaprodine.[1]
Tests in rats showed alphaprodine to be 97% the strength of morphine via the subcutaneous route and 140% the strength of methadone orally.[1] Betaprodine was 550% as strong as morphine SC, and the laevorotary cis isomer was 350% as strong, and the dextrorotary cis isomer was 790% was strong.[1] Betaprodine by mouth was 420% as strong as methadone, and the cis form was 390% for the laevo and 505% for the dextro isomers.[1]
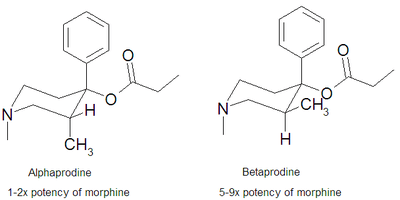
Alphaprodine was sold under several brand names, mainly Nisentil and Prisilidine. It was mainly used for pain relief in childbirth[5] and dentistry,[6] as well as for minor surgical procedures. Alphaprodine has a duration of action of 1 to 2 hours and 40 to 60 mg is equal to 10 mg of morphine via the subcutaneous route.
Prodine has similar effects to other opioids, and produces analgesia, sedation and euphoria. Side effects can include itching, nausea and potentially serious respiratory depression which can be life-threatening. Respiratory depression can be a problem with alphaprodine even at normal therapeutic doses.[7] Unlike pethidine, prodine does not produce toxic metabolites and is therefore more suitable for high-dose therapy.
Regulation
Alphaprodine has a DEA ACSCN of 9010 and 2013 manufacturing quota of 3 grams; betaprodine has an ACSCN of 9611 and a 2 grams quota.
See also
References
- Reynolds & Randall (1957). Morphine & Allied Drugs. pp. 310–312.
- Beckett AH, Walker J (1955). "The configuration of alphaprodine and betaprodine". Journal of Pharmacy and Pharmacology. 7 (1): 1039–1045. doi:10.1111/j.2042-7158.1955.tb12115.x. PMID 13278850.
- John Bedford Stenlake. Foundations of Molecular Pharmacology. ISBN 978-0-485-11171-2.
- Fung DL, Asling JH, Eisele JH, Martucci R (1980). "A comparison of alphaprodine and meperidine pharmacokinetics". Journal of Clinical Pharmacology. 20 (1): 37–41. doi:10.1002/j.1552-4604.1980.tb01664.x. PMID 7358866.
- Burnett RG, White CA (1966). "Alphaprodine for continuous intravenous obstetric analgesia". Obstetrics & Gynecology. 27 (4): 472–477. doi:10.1097/00006250-196604000-00003.
- Carter WJ, Bogert JA (1966). "An effective pre-medication procedure for dental patients". Journal of the Missouri Dental Association. 46 (6): 8–9. PMID 5221807.
- Fuller JD, Crombleholme WR (1987). "Respiratory arrest and prolonged respiratory depression after one low, subcutaneous dose of alphaprodine for obstetric analgesia. A case report". Journal of Reproductive Medicine. 32 (2): 149–151. PMID 3560080.